A site where all Assistant Pharmacist/Pharmacy Assistant/Pharmacy Technician can contribute towards the profession developments, share knowledge, experience and ideas. Feel free to leave your comments.
Thursday, December 8, 2011
OPSYEN UMUR 60 & FAEDAH PERSARAAN
Saturday, July 30, 2011
HISTORY OF PHARMACY
History of pharmacy
.jpg)
Although people have been using medicinal substances to treat themselves for as far back as there have been people, the pharmacy profession has more recent origins. Nevertheless, its roots can be found over 4000 years ago.
Pharmacy’s roots
The history of pharmacy in Britain
Some key dates in pharmacy history
| 1820 | The alkaloid quinine was first extracted from the bark of cinchona trees by two French chemists, Pierre Joseph Pelletier and Joseph Biename Caventou. | |
| 1874 | Diamorphine or Heroin was first synthesised from morphine. | |
| 1883 | First edition of The Extra Pharmacopoeia published, edited by William Martindale and Dr Wynn Westcott. | |
| 1899 | Aspirin, was launched by the German company. | |
| 1910 | Salvarsan, the first 'magic bullet' drug, effective against syphilis was discovered by Paul Ehrlich and Dr Sahachiro Hata. | |
| 1915 | Medicine stamp duty was doubled as a wartime fundraiser. | |
| 1917 | The Venereal Disease Act prohibited the advertising of medicines for VD and selling mixtures containing scheduled substances. It introduced the concept of 'prescription only' medicines. | |
| 1922 | The Dangerous Drugs Act regulated the import and sale of potential 'drugs of addiction', including the derivatives of opium, cocaine and cannabis so widely used in proprietary remedies. | |
| 1928 | Penicillin discovered by Alexander Fleming. | |
| 1938 | The Food and Drugs Act prohibited the adulteration and mislabeling of drugs. | |
| 1939 | The Cancer Act restricted the advertisement of products claiming to treat cancer. | |
| 1940 | Under the Finance (No. 2) Act purchase tax was imposed on a range of goods including most drugs and medicines. | |
| 1941 | The Pharmacy and Medicines Act repealed the old medicine stamp duty. It forbade the general advertisement of products claiming to treat a number of specific illnesses including Bright's disease, cataract epilepsy and TB, or to be effective in procuring an abortion. For the first time manufacturers were required to list the active ingredients of products on their packaging. | |
| 1948 | The National Health Service made prescription medicine available to all. Until the introduction, in the 1950s, and subsequent hefty increasing of prescription charges, proprietary medicines were no longer seen as a cheap alternative to seeing the doctor. | |
| 1961 | Ibuprofen was first synthesised by a team at the Boots Pure Drug Company in December. | |
| 1964 | Introduction of Adverse Drug Reaction 'yellow card' scheme in reponse to the thalidomide tragedy of 1961. |
Tuesday, July 19, 2011
Friday, July 8, 2011
Resistance Exercise Promotes Release of Neurotrophic Factors
Medscape Medical News from the:
Jennifer MontiJune 4, 2010 (Baltimore, Maryland) — New research presented here at the American College of Sports Medicine 57th Annual Meeting shows that circulating levels of brain-derived neurotrophic factor(BDNF) increase after intense resistance training, which might explain why exercise is effective at protecting and improving brain function.The incidence of neurodegenerative diseases, like Alzheimer's disease, is lower in people who participate in endurance exercise on a regular basis, noted Joshua Yarrow, PhD, lead researcher and postdoctoral associate at the University of Florida in Gainesville.He and his colleagues evaluated the effect of resistance training, including weightlifting, on levels of circulating neuroprotective molecules like BDNF.They enrolled 20 college-aged men in a trial to determine whether repeated resistance exercise resulted in increased circulating BDNF at 1, 30, and 60 minutes after exercise. Serum BDNF was 23,304 ± 1,835 pg/mL at rest and increased 32% 1 minute after exercise (P < .05). Serum BDNF levels returned to baseline within 30 minutes and was 41% below resting levels 60 minutes after exercise (P < .01).The study reveals that resistance exercise induces a robust transient increase in circulating BDNF concentrations. It has been shown that during endurance training, BDNF is produced in the brain. But BDNF is produced in many tissues, including musculoskeletal tissue and endothelial tissue. The production of BDNF peripherally with resistance training, which might then affect the brain, is important because we know that BDNF crosses the blood–brain barrier and can act within the brain," Dr. Yarrow said."Resistance exercise may essentially feed BDNF to the brain, which is why we see an increase in circulating levels immediately postexercise and then a decrease in protein within an hour," he added."This is important because we may be able to say that endurance exercise is not necessary for everyone who seeks the neuroprotective benefits of exercise. We may be able to tailor resistance training programs to optimize the exposure of neural tissue to neurotrophic factors like BDNF," Dr. Yarrow explained.J. Carson Smith, PhD, associate professor at the University of Wisconsin, Milwaukee, offered a second interpretation of what happens to the BDNF that is released after resistance training. "In addition to the possibility that the BDNF circulates to the brain and crosses the blood–brain barrier to act in target tissues in the brain, it is possible that the BDNF is released peripherally and then is simply cleared from the circulation after a relatively short amount of time."These findings have implications for the understanding of how both endurance and resistance exercise can lead to the release of neuromodulators that optimize the function of the nervous system from multiple perspectives, Dr. Smith said.Increasing the presence of neurotrophins can have a positive effect on cognition, mood, emotions, and several other domains of brain function. Additional studies may focus on maximizing BDNF release from the peripheral tissue and studying the use of exogenous BDNF as a neuroprotectant for clinical use, the researchers said.Dr. Yarrow and Dr. Smith have disclosed no relevant financial relationships.American College of Sports Medicine (ACSM) 57th Annual Meeting: Abstract 735. Presented June 2, 2010
Monday, July 4, 2011
Practical Knowledge in Antiplatelet Therapy
From Medscape Education Cardiology
John R. Fanikos, RPh, MBA
Acute coronary syndrome (ACS) is a broad term describing the spectrum of clinical symptoms that reflect acute myocardial ischemia or reduced blood supply to the heart. Urgent revascularization is now recognized as the cornerstone of ACS management. Blood flow can be restored through a narrowed or obstructed blood vessel with either coronary artery bypass graft (CABG) surgery or, more commonly now, using a percutaneous coronary intervention (PCI) procedure. In patients stricken with myocardial infarction (MI), PCI reduces mortality, reinfarction, stroke, recurrent ischemia, and hospitalization when compared with conservative medical management strategies.[1-3]
The introduction of drug-eluting stents, compared with bare-metal stents, has reduced the need for target vessel revascularization.[4] However, these procedures and the devices, coupled with a persistent platelet hyperactive state associated with ACS, place patients at additional risk for ischemic events that extends long after clinical stabilization and hospital discharge. To combat this, the use of dual-antiplatelet therapy with complimentary mechanisms of action (most commonly aspirin and clopidogrel) is now a well-established strategy for improving outcomes in both short-term hospital-based invasive procedures[5,6] as well as in the ambulatory setting[7,8] for long-term prevention of secondary cardiovascular events. However, improvements in these therapies are needed because there are patients who will still develop atherothrombotic events while receiving currently available antiplatelet agents. The long-term morbidity and mortality after hospitalization remains significant. The recent introduction of prasugrel, as well as the promising investigational agents elinogrel and ticagrelor offer hope for improving care in patients suffering with cardiovascular disease. Pharmacists are uniquely positioned to interact with patients and their families to provide medication information, identify adverse reactions, and ensure compliance to optimize antiplatelet therapy.
Atherosclerosis is a systemic process in which lipids accumulate in the blood vessel walls and gradually develop into plaques that narrow the lumen and alter blood flow. Autopsy and ultrasound studies have shown that these atherosclerotic plaques are widely distributed throughout the body's blood vessels and become pathological in a variety of medical conditions (Figure 1).[9]
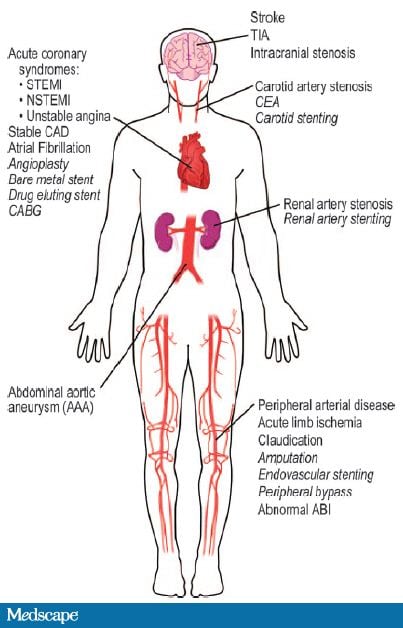
Figure 1. Clinical manifestations of atherosclerotic plaques. From Meadows TA, et al. Circ Res. 2007;100:1261-1275.[15]
In patients with heart disease, these plaques are located diffusely along the coronary arteries and consist of a lipid-rich core and a sclerotic, collagen-rich hard cap. The core is composed primarily of cholesterol and is normally not exposed to blood flow. However, if a plaque destabilizes and ruptures, the lipid components are exposed to blood, and thrombus formation ensues (Figure 2).[10]
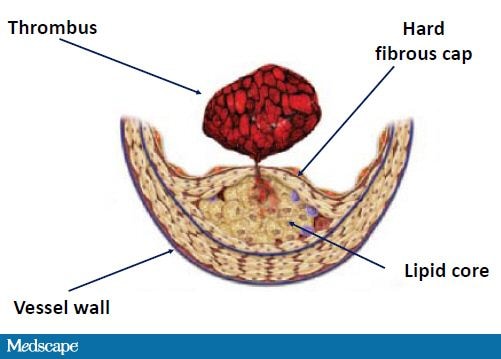
Figure 2. Rupture of atherosclerotic plaque. From Libby P, et al. Circulation. 2005;111:3481-3488.[9]
Luminal thrombosis can obstruct blood flow totally or intermittently and gives rise to ACS with symptoms that patients often describe as chest pressure, squeezing, fullness, or pain. This discomfort may also radiate to the neck, jaw, shoulder, back, or arm.[11] Along with symptoms of chest pain, the diagnosis of ACS is based on changes in the electrocardiogram (ECG) and the presence of biomarkers (creatine kinase-MB and troponin) in the blood that indicate heart muscle damage (Figure 3). An MI can be classified as an ST-elevation MI (STEMI) or a non-ST-elevation MI (non-STEMI) based on ECG changes. Ultimately the clinical presentation and outcome will depend on the severity and duration of myocardial ischemia generated by thrombosis around the culprit lesion.
Role of Platelets
Platelets play a pivotal role in hemostasis, the process of stopping bleeding. While platelets are key to physiologic blood vessel repair, they also have a prominent role in the development of ACS and stroke.[12]Platelets are produced in the bone marrow and released, where they normally circulate in a disc-shaped resting state. A mature platelet has an expected lifespan of 7-10 days. In response to injury, platelets adhere, become activated, and aggregate with one another, producing a thrombus. The generation of a platelet-dependent thrombus requires 3 steps: (1) platelet adhesion; (2) activation, additional recruitment, and aggregation of platelets; and (3) thrombus stabilization.[13]
Platelets roll, spread, and adhere over the damaged vessel wall to form a platelet monolayer. Adhesion results from the interaction of the glycoprotein (GP) receptors on the platelet surface with von Willebrand factor, a circulating plasma protein, and GP receptors and collagen at the site of vascular injury. Platelet activation occurs through 2 adenosine diphosphate (ADP) receptors, P2Y1 and P2Y12, on the platelet surface.[13-15] ADP binding to the P2Y1 receptor results in platelet shape change and rapid reversible platelet aggregation (Figure 5). ADP binding to the P2Y12 receptor signals the release of platelet storage granules containing ADP, thromboxane A2 (TXA2), and other factors. This serves not only to recruit and activate additional platelets but also to amplify platelet aggregation induced by other agents, including thrombin, serotonin, and TXA2. These substances serve to heighten inflammation, attract other mediators to the local area, and enhance the adhesive properties of the vessel wall. Most important, this platelet procoagulant activity converts the GP IIb/IIIa receptor to an active form on the platelet surface. In the final step, activated platelets connect platelet to platelet, bridging via the GP IIb/IIIa receptors and fibrinogen, thereby stabilizing the thrombus.
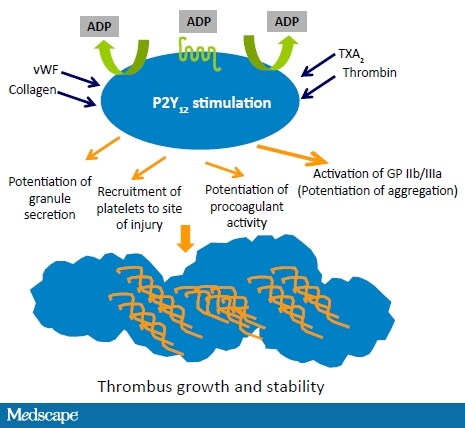
Figure 5. Platelet surface interactions and thrombus formation. From Angiolillo DJ, et al. Circ J. 2010;74:597-607.[13]
In summary, repeated episodes of platelet activation, subsequent thrombus formation, and fragmentation occur at the plaque rupture site.[12] These hemostatic processes contribute to the development of ACS.
Antiplatelet Agents
Aspirin
Aspirin, or acetylsalicylic acid, blocks the platelet activation step of thrombus formation. Derived from the bark of the white willow tree, acetylsalicylic acid has been used pharmacologically since the middle of the 19th century.[16] Aspirin irreversibly inhibits both forms of cyclooxygenase enzymes (COX-1 and COX-2), reducing the formation of prostaglandins and TXA2. The prostaglandin products of COX enzyme activity provide protection from gastrointestinal mucosal injury. Aspirin's irreversible antiplatelet effect lasts for the life of a platelet at doses as low as 30 mg.[17] Larger doses (> 3000 mg daily) are required to inhibit COX-2 and produce systemic anti-inflammatory effects. Consequently, there is a 50- to 100-fold variation in the daily doses of aspirin required to suppress inflammation and inhibit platelet function.
In patients with cardiovascular disease, aspirin effectively reduces the risk for recurrent events by about 30%.[18,19] When studies in patients with MI were combined, aspirin treatment reduced the relative risk for nonfatal MI by 28%, vascular death by 15%, and overall mortality by 11%. Aspirin is effective in a broad range of dosages, 75-325 mg daily. For treatment of ACS in patients undergoing PCI, it is recommended that a full dose (325 mg) of aspirin be given in the acute hospital setting for 1 month, and then the dose may be reduced to between 81 mg and 150 mg, taken indefinitely.[5,6]
Aspirin, however, does not prevent all thrombotic events from recurring. This has led to the identification of patients with aspirin resistance, which may affect as many as 40% of patients.[20] In clinical trials, aspirin resistance has been associated with an increased risk for death, ACS, or new cerebrovascular event.[21] Aspirin resistance is the inability of aspirin to reduce platelet production of TXA2 and the steps of platelet activation and aggregation. Laboratory and platelet function tests measuring TXA2 production and TXA2 activity can detect this condition, but the diagnosis, definition, and testing have not been standardized. A number of potential causes of aspirin resistance have been hypothesized, including inadequate dose, drug interactions, genetic variation in COX-1 enzyme activity and TXA2 biosynthesis, and increased platelet turnover.
Aspirin's most common adverse effects are gastrointestinal (GI) (indigestion, nausea, heartburn, and constipation). These events are usually dose related and can be minimized by using enteric-coated, buffered formulations or taking aspirin with meals.[22] Bleeding is the most serious adverse effect of long-term aspirin therapy, with cerebral bleeding (hemorrhagic stroke, intracranial hemorrhage) and GI bleeding considered major adverse events. Combined trial results suggest these complications are infrequent, with GI bleeding events (about 1 event in 100 patients) occurring more often compared with cerebral bleeding (about 1 event per 1000 patients).[23,24]
P2Y12 Antagonists
ADP receptor antagonists exert their pharmacologic action by inhibiting the P2Y12 receptor on the platelet surface, preventing ADP-induced fibrinogen binding to platelets, a necessary step in the platelet aggregation process. There are 3 FDA-approved P2Y12 antagonists (ticlopidine, clopidogrel, and prasugrel) and 2 agents in clinical development (ticagrelor, elinogrel) (Table 1). While these agents have similar mechanisms of action, they vary widely in their structure, receptor site action, and pharmacokinetics.
Table 1. Pharmacokinetic and Pharmacodynamic Properties of P2Y12 Inhibitors
| Ticlopidine | Clopidogrel | Prasugrel | Ticagrelora | |
| Trade name | Ticlid® | Plavix® | Effient® | Brilinta® |
| Route | Oral | Oral | Oral | Oral |
| Chemical structure | Thienopyridine | Thienopyridine | Thienopyridine | Cyclopentyl-triazolo-pyrimidine |
| Receptor binding | Irreversible | Irreversible | Irreversible | Reversible |
| Pro-drug | Yes | Yes | Yes | No |
| Cytochrome P450 metabolism | CYP 3A4 | CYP 1A2, 2B6, 2C19, 2C9, 3A4, 3A5 | CYP 3A4, 2B6, 2C9, 2C19 | CYP 3A4 |
| Clearance | Renal 60% Fecal 23% | Renal 50% Fecal 46% | Renal 68% Fecal 27% | Fecal 99% Renal <1% |
| Enteral bioavailability | 80% | 50% | 80% | Not reported |
| Time to peak plasma concentration | 1-3 hrs | 1 hr | 30-60 mins | 1.5-3 hrs |
| Time to peak platelet inhibition | 2-5 days | 300 mg LD: 6 hrs 600 mg LD: 2 hrs | 1-2 hrs | 2 hrs |
| Plasma half life | 12 hrs | 30 minsb | 3.7 hrsb | 6-12 hrs |
| Time to steady state | 2-5 days | 3-7 days | 2-4 days | 2-3 days |
| Duration of antiplatelet effect | 7-10 days | 7-10 days | 7-10 days | 1 day |
| Significant drug interactions or genetic polymorphisms | Yes | Yes | No | No |
| FDA indication | 1. To reduce the risk for thrombotic stroke (fatal or nonfatal) in patients who have experienced stroke precursors, and in patients who have had a completed thrombotic stroke 2. As adjunctive therapy with aspirin to reduce the incidence of subacute stent thrombosis in patients undergoing successful coronary stent implantation | 1. For patients with ACS, including patients who are to be managed medically and those who are to be managed with coronary revascularization 2. Recent MI, recent stroke, or established peripheral arterial disease | 1. To reduce the rate of thrombotic cardiovascular events (including stent thrombosis) in patients with ACS who are to be managed with PCI | Not approved for use in the United States |
| ACS = acute coronary syndromes; CYP = cytochrome P; FDA = US Food and Drug Administration; LD = loading dose; MI = myocardial infarction; PCI = percutaneous coronary intervention a. Investigational agent b. Active metabolite half-life Ticlid®: Roche Laboratories; Plavix®: sanofi aventis/Bristol-Myers Squibb; Effient®: Daiichi Sankyo, Inc./Lilly USA; Brilinta®: AstraZeneca. | ||||
Ticlopidine
Ticlopidine, a first-generation thienopyridine, played an important historical role in patients undergoing PCI and coronary artery stenting for management of coronary artery disease. With early use of these stents, thrombosis was a common complication, occurring in as many as 20% of patients.[25] Several studies evaluated the use of aspirin alone, anticoagulant therapy, and ticlopidine with and without aspirin.[25,26] The important findings showed that antiplatelet therapy reduced cardiovascular complications (death, MI, and stent thrombosis) with less bleeding than anticoagulation with warfarin. Furthermore, dual antiplatelet therapy with aspirin and a P2Y12antagonist acts synergistically, reducing cardiovascular events greater than either agent alone. Ticlopidine, however, was associated with a rare but life-threatening blood disorder, thrombotic thrombocytopenic purpura (TTP).[27] Subsequent clinical and registry studies showed clopidogrel was better tolerated, produced fewer side effects, and had better efficacy.[28] Clopidogrel therefore replaced ticlopidine as the standard antiplatelet regimen.
Clopidogrel
Clopidogrel has a long history of providing benefit in patients with cardiovascular disease. The CAPRIE (Clopidogrel vs Aspirin in Patients at Risk of Ischemic Events) study compared clopidogrel vs aspirin therapy in patients with recent MI, ischemic stroke, or symptomatic peripheral arterial disease with the intent of preventing recurrent cardiovascular events. Compared with aspirin, clopidogrel was associated with an 8.7% relative risk reduction in the combined events of vascular death, MI, or ischemic stroke (intent-to-treat analysis).[29]Clopidogrel also reduced the risk for rehospitalization for ischemic events vs aspirin with no major differences in adverse events between the 2 therapies. The results from CAPRIE supported clopidogrel as the alternative antiplatelet agent of choice for patients with aspirin intolerance.
The CURE (Clopidogrel in Unstable angina to prevent Recurrent Events) study was performed in patients with ACS without ST-segment elevation.[30] Patients received clopidogrel (300 mg immediately followed by 75 mg once daily) plus aspirin or aspirin alone daily. Over 12 months there was a relative risk reduction of 20% in the composite outcome of nonfatal MI, stroke, or cardiovascular death in clopidogrel plus aspirin-treated patients. In the substudy of patients treated with PCI (PCI-CURE), there was a 30% relative risk reduction in the composite of cardiovascular death, MI, or urgent target-vessel revascularization (within 30 days of PCI) and a 31% relative risk reduction in cardiovascular death or MI overall (before and after PCI) in patients who received clopidogrel plus aspirin.[31] Findings from the CREDO (Clopidogrel for the Reduction of Events During Observation) trial supported the beneficial effect of clopidogrel plus aspirin therapy in the long-term management of patients undergoing planned PCI.[32] At 1 year, clopidogrel plus aspirin reduced the relative risk for death, MI, or stroke by 27%. CREDO also provided insight into the most appropriate timing of clopidogrel administration prior to PCI. The COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) and CLARITY-TIMI 28 (CLopidogrel as Adjunctive ReperfusIon TherapY-Thrombolysis In Myocardial Infarction 28) studies focused on the early medical management of MI in the hospital, combining clopidogrel and aspirin with thrombolysis.[33,34] In both studies, clopidogrel and aspirin reduced the combined adverse cardiovascular outcomes of death, MI, stroke, or occluded infarct-related coronary vessels.
There have been recent trials, however, in which the benefit of clopidogrel has not been universal. In the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) study, outcomes were compared in patients with stable cardiovascular disease or asymptomatic patients with multiple cardiovascular risk factors.[35] The combination of aspirin plus clopidogrel did not reduce adverse cardiovascular outcomes when compared with aspirin alone and was associated with an increased risk for bleeding. The CURRENT-OASIS 7 (Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions 7) demonstrated no difference in adverse cardiovascular events at 30 days between patients with ACS who received double-dose clopidogrel vs standard-dose clopidogrel.[36] In patients in whom platelet function is studied, those with a high residual platelet reactivity have a higher risk for cardiovascular events after stent implantation. In the GRAVITAS trial (Gauging Responsiveness With A VerifyNow Assay – Impact on Thrombosis and Safety), patients were treated with either high-dose clopidogrel (150 mg daily) or standard-dose clopidogrel (75 mg daily). The composite endpoint of death, MI, and stent thrombosis was the same (2.3%) in both groups at 6 months. This study suggests alternative treatment strategies to clopidogrel may be necessary to improve patient outcomes.[37]
In addition to factors such as dosing and timing of intervention, the potential benefit of clopidogrel treatment is related to its metabolism. The phenomenon of "clopidogrel resistance" has led to efforts investigating reasons for the wide variability in patient response including the pharmacodynamics of this agent.[38]
Clopidogrel, a second-generation thienopyridine, is a prodrug for which hepatic metabolism is essential to the generation of its active metabolite. Approximately 85% of a clopidogrel dose is metabolized by blood esterases into an inactive metabolite. The remaining clopidogrel undergoes a 2-step process involving multiple cytochrome P450 enzymes. In the first metabolic step, clopidogrel is catalyzed by 3 enzymes (CYP1A2, CYP2B6, and CYP2C19) and leads to an inactive metabolite (2-oxo-clopidogrel). The second step requires 4 enzymes (CYP2B6, CYP2C9, CYP2C19, and CYP3A4) and produces an active metabolite.[39] Variations or polymorphisms in the gene that codes for CYP2C19 appears to be the most important. A reduced function gene, which is present in 15%-30% of ACS patients, is associated with reduced concentrations of active drug, a 50% higher risk for death, MI, and stroke, as well as a 3-fold higher risk for stent thrombosis.[40] An enhanced function gene is associated with rapid metabolism, heightened antiplatelet activity, and an increased bleeding risk.[41] In response, the US Food and Drug Administration (FDA) has incorporated a boxed warning alerting clinicians of the "diminished effectiveness in poor metabolizers" and recommendations to consider alternative agents.
P-glycoprotein, a membrane-associated protein responsible for drug transport, affects clopidogrel absorption. Genetic variants can alter clopidogrel concentrations, reduce platelet inhibition, and compromise efficacy. A recent study has shown ACS patients with this specific genotype undergoing PCI are at higher risk for death, MI, and stroke.[42]
Drug interactions may affect clopidogrel efficacy. When coadministered, statins that are metabolized by CYP450 enzymes, such as atorvastatin or simvastatin, may compete with clopidogrel, reducing its activation and attenuating its antiplatelet effect.[43] With a similar mechanism to statins, proton-pump inhibitors, which are the preferred agents for aspirin-associated GI injury, have been shown to reduce the antiplatelet effect and efficacy of clopidogrel.[44,45] While trials have not confirmed these interactions clinically,[43,46] the FDA issued a public health advisory warning, and reissued a reminder warning in October 2010, that concomitant use of clopidogrel and omeprazole can significantly reduce antiplatelet activity.[47]
While genetic testing is available, controversy exists over its value and role.[48] Point-of-care genetic testing with a rapid turnaround (3-8 hours) has been limited to research studies. Furthermore, identifying the CYP2C19 gene only explains about 10% of the variation in clopidogrel response.[49] Other environmental and clinical factors can affect clopidogrel metabolism and platelet function (Figure 6). Recognizing that one dose may not fit all, clinicians are faced with implementing untested strategies such as increasing the clopidogrel daily dose or adding additional weak-acting antiplatelet medications like dipyridamole or cilostazol. An alternative option is to consider the use of novel P2Y12 receptor inhibitors.
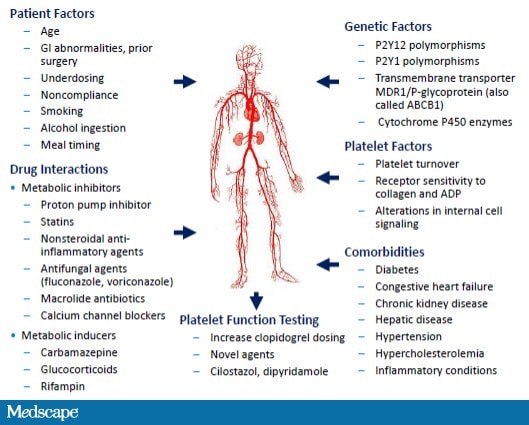
Figure 6. Factors affecting clopidogrel response.
Prasugrel
Prasugrel, like clopidogrel, is a thienopyridine but with 10-fold greater anti-P2Y12 receptor inhibitory activity.[50,51]Prasugrel has a more rapid onset, irreversibly binds to the P2Y12 receptor, and inhibits platelet activity more consistently and extensively than do standard and higher doses of clopidogrel (Table 1).[52-54] Prasugrel is a prodrug, metabolized in one step via the CYP450 system, and is not susceptible to genetic polymorphisms and drug-drug interactions that are associated with adverse cardiovascular outcomes (Table 1).[55]
In the Trial to Assess Improvement in TRITON-TIMI 38 (Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction) study, ACS patients scheduled for PCI were treated with either clopidogrel (300 mg loading dose, then 75 mg daily) or prasugrel (60 mg loading dose, then 10 mg daily) and followed over the subsequent 6-15 months.[56] In the prasugrel-treated patients, there was a 19% relative risk reduction in the primary efficacy endpoint (death from cardiovascular causes, nonfatal MI, and nonfatal stroke) compared with clopidogrel (Figure 7). Additionally, the frequency of both early and late stent thrombosis was reduced by 52%. This benefit was magnified in high-risk groups, such as patients with diabetes mellitus or STEMI, and was apparent as early as day 3.[56,57] The benefit of prasugrel vs clopidogrel was consistent across subsets of diabetes patients (insulin- and non-insulin-dependent).
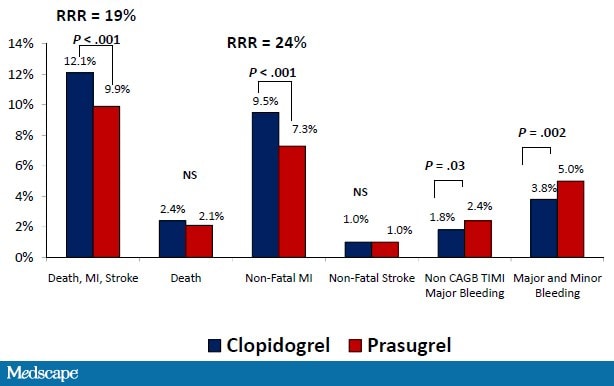
Figure 7. TRITON-TIMI 38 patient outcomes. RRR = relative risk reduction; NS = not significant. From Wiviott SD, et al. N Engl J Med. 2007;357:2001-2015.[56]
There was, however, a sacrifice in that prasugrel was associated with a statistically significant increase in non-CABG-related TIMI major bleeding, TIMI major bleeding and minor bleeding, and fatal bleeding.[56] The risk for bleeding was related to diabetes status with statistically significant excess risk with prasugrel vs clopidogrel in patients without diabetes, but not in patients with diabetes.[57] Some groups appeared to have less clinical efficacy and were particularly susceptible to bleeding. These groups included the elderly (age ≥ 75 years), the underweight (< 60 kg), and patients with a previous cerebrovascular event (stroke or transient ischemic attack). In the small number of patients with a previous stroke or transient ischemic attack, prasugrel significantly increased the rate of intracranial hemorrhage.
The prolonged, irreversible antiplatelet effect is of concern when patients need urgent surgery, such as CABG, that cannot be postponed. Treatment-related hemorrhagic complications have been strongly linked to subsequent mortality in patients with cardiovascular disease.[58] Overall, the increase in prasugrel-related bleeding may partially offset the benefits from prevention of MI and stent thrombosis. As a result, there were no statistical differences between prasugrel and clopidogrel in all-cause mortality (3.0% vs 3.2%) at 15 months. However, stratifying by diabetes status showed a statistically greater net clinical benefit (composite cardiovascular death, nonfatal MI, stroke, nonfatal TIMI major bleed not related to CABG) with prasugrel among patients with diabetes.[57]
Prasugrel was approved by the FDA in July 2009 with a boxed warning incorporated into its labeling alerting clinicians that it is contraindicated in patients with prior cerebrovascular events and to avoid its use in those at a high risk for bleeding (age ≥ 75 years, body weight < 60 kg, those taking concomitant medications that increase bleeding risk, and those likely to undergo CABG surgery). The dosing instructions also include recommendations to consider reducing the maintenance dose to 5 mg daily.[59]
Ticagrelor
Ticagrelor, unlike clopidogrel and prasugrel, is a reversible, direct-acting, oral antagonist of the P2Y12 receptor. It is a new class of agents, the cyclo-pentyl-triazolo-pyrimidines. Ticagrelor provides faster, greater, and more consistent P2Y12 inhibition than clopidogrel (Figure 8).[60-63]
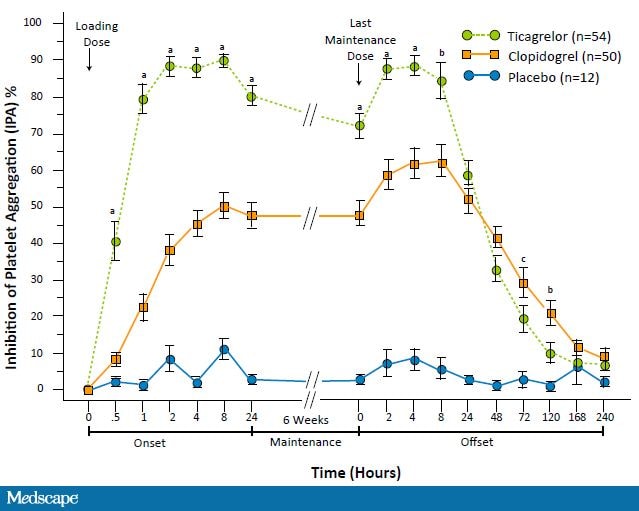
Figure 8. Antiplatelet agent pharmacokinetics inhibition of platelet activity: ticagrelor vs clopidogrel vs placebo. a.P<.0001; b. P<.005; c. P<.05. From Gurbel PA, et al. Circulation. 2009;120;2577-2585.[62]
Peak platelet inhibition, as measured by inhibition of platelet aggregation, is seen within 2 hours after ticagrelor administration as compared with 24 hours with clopidogrel. A greater platelet inhibition is sustained with maintenance dosing. After 14 and 28 days of dosing, ticagrelor inhibited 90%-95% of platelet activity as compared with 60% with clopidogrel. Ticagrelor's offset is faster than clopidogrel with a significantly lower platelet inhibition seen 72 hours after the last dose. The unique feature of reversible receptor binding is evident at 24 hours. This decrease in platelet aggregation necessitates every-12-hours dosing. Despite this reduction, platelet inhibition is still greater with ticagrelor than with clopidogrel, suggesting omitted doses may not have dire consequences.
Ticagrelor is metabolized by the CYP450 system to an equipotent active metabolite, which is further metabolized via glucuronidation. Since less than 1% of the ticagrelor dose is found in the urine, dose adjustments may be warranted in hepatic but not in renal dysfunction.[63,64] Studies have confirmed that the genetic polymorphisms that affect clopidogrel absorption and active metabolite generation do not affect ticagrelor pharmacodynamics.[65]Nearly all patients who do not respond to clopidogrel, when switched to ticagrelor had a reduction in the platelet activity level that is associated with ischemic risk.[66]
In the PLATO (PLATelet inhibition and patient Outcomes) study, patients with ACS were treated with either clopidogrel (300 mg loading dose with an additional 300 mg for patients undergoing PCI, then 75 mg daily) or ticagrelor (180 mg loading dose with an additional 90 mg for patients undergoing PCI, then 90 mg twice daily) and followed over the subsequent 12 months.[67] In the ticagrelor-treated patients, there was a 16% relative risk reduction in the primary efficacy endpoint (death from cardiovascular causes, MI, and stroke) (Figure 9).
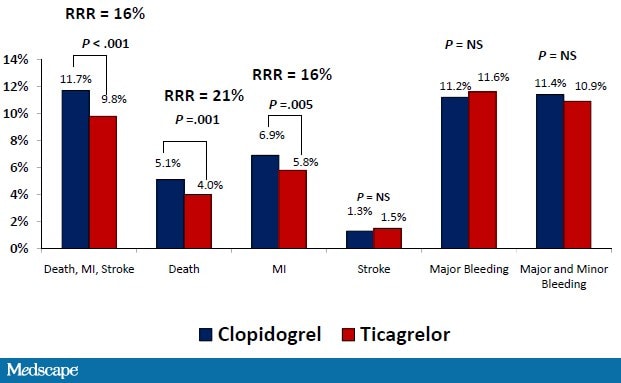
Figure 9. PLATO patient outcomes. RRR = relative risk reduction; NS = not significant. From Wallentin L, et al.N Engl J Med. 2009;361:1045-1057.[67]
There was a 22% relative risk reduction in all-cause mortality, and the risk for definite stent thrombosis was reduced by 33%. These benefits were maintained in patients managed with PCI, where there was no significant difference in the primary safety endpoint of major bleeding or the combination endpoint of major and minor bleeding between the ticagrelor and clopidogrel groups.[68] However ticagrelor was associated with a higher rate of major or minor bleeding not related to CABG. Renal dysfunction in ACS patients is associated with a worse prognosis and an increased bleeding risk.[69] Ticagrelor was more effective in reducing cardiovascular events than clopidogrel regardless of renal function, with greater benefit in those with poor renal function, and without any need for dose reduction to prevent major bleeding.[64]
Bradycardia and transient dyspnea, not associated with adverse changes in cardiac or pulmonary function, are common side effects associated with ticagrelor administration, occurring in 4.4% and 13.8% of patients enrolled in the PLATO study, respectively. Results from the small, 6-week ONSET/OFFSET study indicated that drug discontinuation due to adverse events occurred only slightly more frequently with ticagrelor (7.4%) than with clopidogrel (6.0%).[70] Of the 57 patients taking ticagrelor, 22 (38.6%) experienced dyspnea, compared with 5 taking clopidogrel (9.3%) and 1 in the placebo group (8.3%). Of the 22 patients who developed dyspnea while taking ticagrelor, 3 patients had to discontinue the medication (5.3%). Most of the sustained episodes of dyspnea with the ticagrelor group resolved upon cessation of treatment.
The major PLATO study finding that ticagrelor reduced all-cause mortality has led experts to speculate about possible reasons.[71,72] Some believe it is related to the rapid offset of activity and the ability to transition patients to CABG surgery or better manage bleeding episodes. Because adenosine is involved in numerous biological activities, others have hypothesized that adenosine inhibition may improve myocardial performance, microcirculatory coronary blood flow, cardioprotection from reperfusion injury, and myocyte regeneration. Dyspnea and bradyarrhythmias could be related to the inhibition of adenosine as well.
Ticagrelor is not yet approved in the United States; however, the FDA Cardiovascular and Renal Drugs Advisory Committee voted to recommend its approval for preventing thrombotic events in patients with ACS in July 2010.[73]
Role of the Pharmacist
The pharmacist's role has changed dramatically over the past 30 years, becoming more patient oriented, increasingly providing medication therapy management as part of their professional practices, and integrating into the healthcare delivery team. Because pharmacists are highly accessible and skilled in medication counseling and education, they can play a much needed role in improving access to care, patient satisfaction, clinical outcomes, and reducing costs. Community and clinic-based pharmacists have successfully participated in disease state management including diabetes and hypertension,[74,75] as well as in drug class management such as anticoagulation, immunosuppressive, and antibiotic therapy.[76-78] Similar to these initiatives, a simple 5-step action plan for pharmacists can assist in improving outcomes in those patients requiring antiplatelet therapy (Table 2).
Table 2. Pharmacist Action Plan for Antiplatelet Therapy
| Step | Pharmacist Setting | Pharmacist Action |
| 1 | Transition | Provide patients and family with their antiplatelet therapy treatment plan (medications, doses, duration) at hospital discharge |
| 2 | Continuum | Eliminate delays in prescription filling, ensure prescription is picked up and available for administration, prevent premature discontinuation |
| 3 | Community -- Initiation | Educate patient and family about the medication, compliance, drug-food interactions, and adverse events |
| 4 | Community -- Maintenance | Monitor comorbidities, medication use, refill intervals, and over-the-counter medication purchases and detect drug-related problems |
| 5 | Community -- Outcomes | Critically evaluate with the patient the outcomes of treatment for safety and efficacy and the need for therapy changes |
Hospitalization and subsequent discharge is a hectic, confusing period for many patients that often results in new medications or changes in existing regimens. In this transition, pharmacists can provide patients and their family with the antiplatelet therapy treatment plan (medications, doses, and duration) at hospital discharge. This education is now a requirement with oral anticoagulation and makes sense to extend to antiplatelet therapy whenever possible.[79]
Surprisingly, 1 out of 6 patients will delay filling their antiplatelet medication prescription after stent insertion.[80]Even with a prescription benefit, as many as 1% of patients will abandon their index prescription for an antiplatelet agent.[81] Recent registry data show that only 79% of patients with risk factors for atherothrombosis or established cardiovascular disease received any form of antiplatelet therapy.[82]
These delays are associated with an increased risk for death and MI. Physicians have been alerted to the importance of premature discontinuation of dual antiplatelet therapy and stent thrombosis.[83] While other prescriptions were not associated with adverse outcomes, this problem demonstrates the discontinuity of care among care providers. It also highlights a role for pharmacists to ensure patients have access to medications. Pharmacists can facilitate continuity by providing the prescription at discharge or by having selected a pharmacy care provider that has the prescription waiting and, probably most important, making sure the medication is taken home and available for administration.
With initiation of therapy, community pharmacists can provide additional counseling on the antiplatelet medication, the importance of compliance, drug-drug and drug-food interactions, and adverse events. Pharmacist medication and medication profile review, patient counseling, and follow-up patient contact 3-5 days after hospital discharge facilitates clarifying medication regimens, reviewing indications, directions, and potential side effects. It also provides an opportunity to provide physician feedback when necessary. This intervention has been associated with a reduction in adverse events 30 days after hospital discharge.[84]
While it is easy to point to genetic causes, genetic variation may explain only 50% of the variation in response to clopidogrel. There are multiple influences on a medication's response including genetics, concomitant disease processes, other medications, foods, age, and lifestyle. All will affect clopidogrel metabolism and platelet function. Similarly, a number of comorbidities including ACS, left ventricular dysfunction, diabetes mellitus, and renal insufficiency will independently alter platelet function. Furthermore, nonadherence may be the major cause of clopidogrel resistance. The overall nonadherence rate with clopidogrel therapy is about 22% of treated patients.[85]Through their interactions, pharmacists are in a position to monitor symptoms associated with comorbidities and lifestyle changes. Routine direct measurements of platelet activity to detect antiplatelet therapy regimen adherence would be expensive and burdensome to the patient.[86] Indirect measurements of adherence such as asking the patient directly; performing tablet counts; providing questionnaires, diaries, or calendars; or engaging caregiver or family support are all effective methods. Pharmacy computer systems and refill records can assist in monitoring medication compliance rather than escalating therapy to more aggressive combination antiplatelet regimens. Interventions to improve adherence include patient education, simplified or cued dosing schedules that surround daily activities (sleep, meals, hygiene, etc.), medication pill boxes or dose organizers, and constant communication to and between providers. Because many factors contribute to medication adherence, a single intervention may not be enough or work for all patients.
Finally, the pharmacist must critically evaluate with the patient the outcomes of treatment for safety, efficacy, and the need for therapy changes. Symptomatic events should be reported back to the physician. The availability of 3 antiplatelet agents provides additional options and makes it possible to individualize antiplatelet therapy. Prasugrel use should be discouraged in patients with, or who have developed, or are faced with, a high bleeding risk. Ticagrelor therapy may be preferred in patients for whom a CABG procedure is deemed probable or for those who are receiving clopidogrel or prasugrel and need elective surgery. Ticagrelor therapy should be discouraged in patients who have restrictive airway disease, cardiac arrhythmias, or compliance issues with a twice-a-day regimen. The pharmacists can assist in tailoring antiplatelet therapy to meet the patient's needs or existing conditions.
Case Study
WL is a 51-year-old white woman who was shoveling snow when she suddenly developed severe chest pain and shortness of breath. Her husband dialed 911, activating the local emergency response system, and an ambulance arrived in 15 minutes. An ECG was interpreted as showing acute ST-segment elevations indicative of MI (Figure 3).
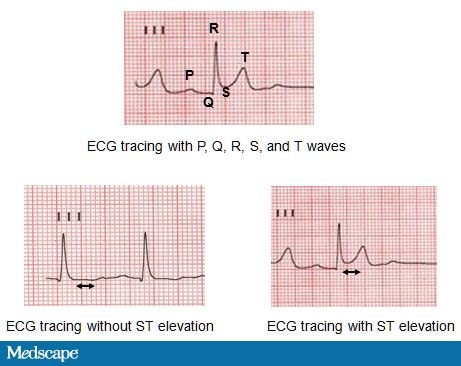
Figure 3. ECG tracings.
WL was transferred to a hospital where coronary angiography showed a 90% occlusion in the right coronary artery. There were also 30% lesions in the left coronary and circumflex arteries. PCI was performed on the right coronary artery with a drug-eluting stent, and blood flow was restored (Figure 4). WL was discharged on a regimen of metoprolol (sustained release) 100 mg daily, aspirin 325 mg daily, clopidogrel 75 mg daily, and atorvastatin 80 mg daily.
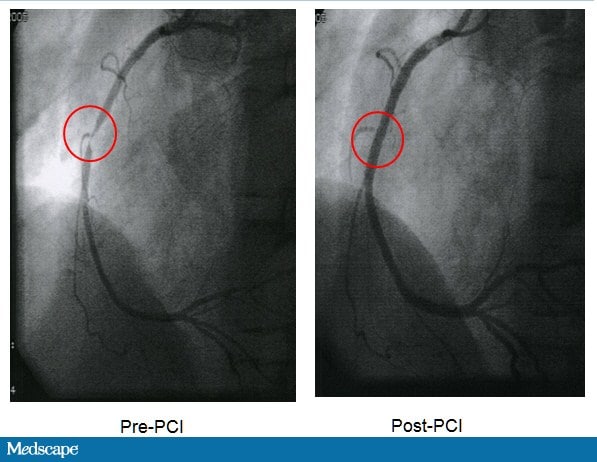
Figure 4. Pre- and post-PCI images.
Six weeks later, WL presented to the emergency department complaining of increasing anginal pain over the last 3 weeks with minimal exertion. She perceived the pain as indigestion and self treated with over-the-counter omeprazole. Today the pain radiated to both arms and shoulders without relief with sublingual nitroglycerin.
An ECG showed ST-depression and T-wave inversions. She admits she has had difficulty in maintaining adherence to her medication regimens. Intravenous nitroglycerin was initiated in the emergency department, and she was transferred to the cath lab. No new lesions were found, but a thrombus was detected in the stent in the right coronary artery. She was transferred to the coronary care unit where a platelet reactivity test revealed that she was resistant to clopidogrel. Omeprazole was ceased, and she was transitioned to prasugrel 10 mg once daily. She was then transferred to a step-down cardiology unit, where a pharmacist provided:
- Medication counseling including verbal and written information about the use of the medications, including how long to take them and what to do if side effects occurred or a dose was missed; and
- Medications organized into a calendar pill box with directions to take them before brushing her teeth every morning.
WL provided feedback that she understood the information and directions, and a time and date for a follow-up phone call was scheduled in 2 weeks to check on the adherence.
Conclusions
Cardiovascular disease, in the form of atherosclerotic plaques, leads to ACS. Antiplatelet therapy is a key component of hospital care, supporting early invasive management, and extends to the home setting to prevent recurrent events. Clopidogrel is hampered by a variable patient response that is related to both genetic and environmental factors. Novel antiplatelet agents represent an advance in pharmacotherapy with more favorable pharmacodynamics and potential improvement in patient outcomes. Prasugrel and ticagrelor may obviate the need for genetic testing that may be necessary with clopidogrel. Pharmacists can play a key role in ensuring prescriptions are filled and compliance to regimens is maintained. They can assist in patient education and be vigilant for and help manage side effects and adverse reactions.
Supported by an independent educational grant from AstraZeneca
LABELS
- AGM PPFM (3)
- Anjakan Paradigma PPF (1)
- Annual General Meeting Malaysian Pharmacy Assistant (2)
- Antiplatelet Theraphy (1)
- Assistant Pharmacist (3)
- Bahagian Farmasi (1)
- Clopidogrel (1)
- Degree in Pharmaceutical Technology (1)
- Dispenser (1)
- Dispensing (1)
- Drug Injury Watch (1)
- Farmasi (3)
- Graduan Diploma KSKB (1)
- Health Centre (1)
- History Of Malaysian Assistant Pharmacy (1)
- History Of Pharmacy Assistant of Malaysia (1)
- Ijazah Sains Farmaseutikal (1)
- Ijazah Teknologis Farmasi (1)
- Juruteknologi Farmasi (1)
- KSKB (1)
- Kursus Paramedik (1)
- massage theraphy (1)
- Mesyuarat Agong Persatuan Penolong Pegawai Farmasi (1)
- Mimpi Penolong Pegawai Farmasi (1)
- P (1)
- Pegawai Farmasi (1)
- Pelatih (2)
- Pembantu Farmasi (30)
- Pembentangan Kertas Kerja (2)
- Penolong Pegawai Farmasi (32)
- Persatuan Penolong Pegawai Farmasi (2)
- Persidangan (1)
- Pharmacist Assistant (24)
- Pharmacist Assistant Conference (2)
- Pharmacy (15)
- Pharmacy Assistant (15)
- Pharmacy Services (6)
- Pharmacy Technician (14)
- Pharmacy Technologist (11)
- Polyclinic (1)
- Post Basic Management (1)
- PPF Dream (1)
- Prasugrel (1)
- Prescription (1)
- Research on Job Satisfaction Among Assistant Pharmacist (1)
- Role Of Pharmacist (1)
- scientific meeting (1)
- Sejarah Penolong Pegawai Farmasi (1)
- Ticlopidine (1)
- Top 50 Pharmacy Blog (1)

