Author: Jose A Hidalgo, MD; Chief Editor: Burke A Cunha, MD
Practice Essentials
Candidiasis is a fungal infection caused by yeasts from the genus Candida. C albicans is the predominant cause of the disease.
Essential update: FDA issues warning for antifungal medication ketoconazole
The FDA announced that clinicians should no longer prescribe ketoconazole (Nizoral, Janssen Pharmaceuticals) tablets as a firstline therapy for any fungal infection, including Candida and dermatophyte infections, because of the risk for severe liver injury, adrenal insufficiency, and adverse drug interactions. The FDA also cautioned that ketoconazole tablets should not be prescribed for any patient with underlying liver disease. The labeling changes do not apply to topical formulations of ketoconazole in creams, shampoos, foams, and gels. Oral ketoconazole is now indicated only for endemic mycoses in patients who fail to respond to or cannot tolerate other treatments.
Ketaconazole tablets were also withdrawn from the market in the European Union in July.[1, 2]
Signs and symptoms
Chronic mucocutaneous candidiasis
Findings reveal disfiguring lesions of the face, scalp, hands, and nails. Chronic mucocutaneous candidiasis is occasionally associated with oral thrush and vitiligo.
Oropharyngeal candidiasis
The patient usually has a history of HIV infection, wears dentures, has diabetes mellitus, or has been exposed to broad-spectrum antibiotics or inhaled steroids. Although patients are frequently asymptomatic, when symptoms do occur they can include the following:
- Sore and painful mouth
- Burning mouth or tongue
- Dysphagia
- Thick, whitish patches on the oral mucosa
Physical examination reveals a diffuse erythema and white patches that appear on the surfaces of the buccal mucosa, throat, tongue, and gums.
The following are the 5 types of OPC:
- Membranous candidiasis - One of the most common types; characterized by creamy-white, curdlike patches on the mucosal surfaces
- Chronic atrophic candidiasis (denture stomatitis) - Also thought to be one of the most common forms of the disease; presenting signs and symptoms include chronic erythema and edema of the portion of the palate that comes into contact with dentures.
- Erythematous candidiasis - Associated with an erythematous patch on the hard and soft palates
- Angular cheilitis - Inflammatory reaction characterized by soreness, erythema, and fissuring at the corners of the mouth
- Mixed - A combination of any of the above types is possible
Esophageal candidiasis
Patients may be asymptomatic or may have 1 or more of the following symptoms:
- Normal oral mucosa - >50% of patients
- Dysphagia
- Odynophagia
- Retrosternal pain
- Epigastric pain
- Nausea and vomiting
Physical examination almost always reveals oral candidiasis.
Nonesophageal gastrointestinal candidiasis
The following symptoms may be present:
- Epigastric pain
- Nausea and vomiting
- Abdominal pain
- Fever and chills
- Abdominal mass (in some cases)
Genitourinary tract candidiasis
The types of genitourinary tract candidiasis are as follows:
- Vulvovaginal candidiasis (VVC) - Erythematous vagina and labia; a thick, curdlike discharge; and a normal cervix upon speculum examination[3]
- Candida balanitis - Penile pruritus and whitish patches on the penis
- Candida cystitis - Many patients are asymptomatic, but bladder invasion may result in frequency, urgency, dysuria, hematuria, and suprapubic pain
- Asymptomatic candiduria - Most catheterized patients with persistent candiduria are asymptomatic
- Ascending pyelonephritis - Flank pain, abdominal cramps, nausea, vomiting, fever, chills and hematuria
- Fungal balls - Intermittent urinary tract obstruction with subsequent anuria and ensuing renal insufficiency
See Clinical Presentation for more detail.
Diagnosis
Diagnostic tests for candidiasis include the following:
- Mucocutaneous candidiasis - For a wet mount, scrapings or smears obtained from skin, nails, or oral or vaginal mucosa are examined under the microscope; a potassium hydroxide smear, Gram stain, or methylene blue is useful for direct demonstration of fungal cells
- Cutaneous candidiasis - Using a wet mount, scrapings or smears obtained from skin or nails can be examined under the microscope; potassium hydroxide smears are also useful
- Genitourinary candidiasis - A urinalysis should be performed; evidence of white blood cells (WBCs), red blood cells (RBCs), protein, and yeast cells is common; urine fungal cultures are useful
- Gastrointestinal candidiasis - Endoscopy with or without biopsy
See Workup for more detail.
Management
- Cutaneous candidiasis - Most localized cutaneous candidiasis infections can be treated with any number of topical antifungal agents (eg, clotrimazole, econazole, ciclopirox, miconazole, ketoconazole, nystatin)
- Chronic mucocutaneous candidiasis - This condition is generally treated with oral azoles
- Oropharyngeal candidiasis - This can be treated with either topical antifungal agents or systemic oral azoles
- Esophageal candidiasis - Treatment requires systemic therapy with fluconazole
- VVC - Topical antifungal agents or oral fluconazole can be used[4]
- Candida cystitis - In noncatheterized patients, Candida cystitis should be treated with fluconazole; in catheterized patients, the Foley catheter should be removed or replaced; if the candiduria persists after the catheter change, then patients can be treated with fluconazole
See Treatment and Medication for more detail.
Image library
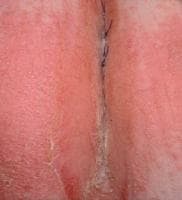 A moist, erosive, pruritic patch of perianal skin and perineum (with satellite pustule formation) is demonstrated in this woman with extensive candidosis. Courtesy of Matthew C. Lambiase, DO
A moist, erosive, pruritic patch of perianal skin and perineum (with satellite pustule formation) is demonstrated in this woman with extensive candidosis. Courtesy of Matthew C. Lambiase, DOBackground
Candidiasis is caused by infection with species of the genus Candida,predominantly with Candida albicans.Candida species are ubiquitous fungi that represent the most common fungal pathogens that affect humans. The growing problem of mucosal and systemic candidiasis reflects the enormous increase in the number of patients at risk and the increased opportunity that exists forCandida species to invade tissues normally resistant to invasion. Candida species are true opportunistic pathogens that exploit recent technological advances to gain access to the circulation and deep tissues.
The increased prevalence of local and systemic disease caused by Candidaspecies has resulted in numerous new clinical syndromes, the expression of which depends primarily on the immune status of the host. Candida species produce a wide spectrum of diseases, ranging from superficial mucocutaneous disease to invasive illnesses, such as hepatosplenic candidiasis, Candidaperitonitis, and systemic candidiasis. The management of serious and life-threatening invasive candidiasis remains severely hampered by delays in diagnosis and the lack of reliable diagnostic methods that allow detection of both fungemia and tissue invasion by Candida species.
Advances in medical technology, chemotherapeutics, cancer therapy, and organ transplantation have greatly reduced the morbidity and mortality of life-threatening disease. Patients who are critically ill and in medical and surgical ICUs have been the prime targets for opportunistic nosocomial fungal infections, primarily due toCandida species. Studies suggest that the problem is not under control and, in fact, show it is worsening. On a daily basis, virtually all physicians are confronted with a positive Candida isolate obtained from one or more various anatomical sites. High-risk areas for Candida infection include neonatal, pediatric, and adult ICUs, both medical and surgical.[5] Candida infections can involve any anatomical structure.
Pathophysiology
Candida species are yeastlike fungi that can form true hyphae and pseudohyphae. For the most part, Candida species are confined to human and animal reservoirs; however, they are frequently recovered from the hospital environment, including on foods, countertops, air-conditioning vents, floors, respirators, and medical personnel. They are also normal commensals of diseased skin and mucosal membranes of the gastrointestinal, genitourinary, and respiratory tracts.
Candida species also contain their own set of well-recognized but not well-characterized virulence factors that may contribute to their ability to cause infection.[6] The main virulence factors include the following:
- Surface molecules that permit adherence of the organism to other structures (eg, human cells, extracellular matrix, prosthetic devices)
- Acid proteases and phospholipases that involve penetration and damage of cell envelopes
- Ability to convert to a hyphal form (phenotypic switching)
As with most fungal infections, host defects also play a significant role in the development of candidal infections. Host defense mechanisms against Candidainfection and their associated defects that allow infection are as follows:
- Intact mucocutaneous barriers - Wounds, intravenous catheters, burns, ulcerations
- Phagocytic cells -Granulocytopenia
- Polymorphonuclear leukocytes - Chronic granulomatous disease
- Monocytic cells -Myeloperoxidase deficiency
- Complement -Hypocomplementemia
- Immunoglobulins -Hypogammaglobulinemia
- Cell-mediated immunity - Chronic mucocutaneous candidiasis, diabetes mellitus, cyclosporin A, corticosteroids, HIV infection
- Mucocutaneous protective bacterial florae - Broad-spectrum antibiotics
Risk factors associated with invasive or systemic candidiasis include the following:[7]
- Granulocytopenia
- Bone marrow transplantation
- Solid organ transplantation (liver, kidney)
- Parenteral hyperalimentation
- Hematologic malignancies
- Foley catheters
- Solid neoplasms
- Recent chemotherapy or radiation therapy
- Corticosteroids
- Broad-spectrum antibiotics
- Burns
- Prolonged hospitalization
- Severe trauma
- Recent bacterial infection
- Recent surgery
- Gastrointestinal tract surgery
- Central intravascular access devices
- Premature birth
- Hemodialysis
- Acute and chronic renal failure
- Mechanical ventilation for longer than 3 days
The first step in the development of a candidal infection is colonization of the mucocutaneous surfaces. All of the factors outlined above are associated with increased colonization rates. The routes of candidal invasion include (1) disruption of a colonized surface (skin or mucosa), allowing the organisms access to the bloodstream, and (2) persorption via the gastrointestinal wall, which may occur following massive colonization with large numbers of organisms that pass directly into the bloodstream.
Frequency
United States
Candida species are the most common cause of fungal infection in immunocompromised persons. Oropharyngeal colonization is found in 30-55% of healthy young adults, and Candida species may be detected in 40-65% of normal fecal florae.
Three of every 4 women experience at least one bout of vulvovaginal candidiasis(VVC) during their lifetime.
More than 90% of persons infected with HIV who are not receiving highly active antiretroviral therapy (HAART) eventually develop oropharyngeal candidiasis (OPC), and 10% eventually develop at least one episode of esophageal candidiasis.[8]
In persons with systemic infections, Candida species are now the fourth most commonly isolated pathogens from blood cultures.[9]
Clinical and autopsy studies have confirmed the marked increase in the incidence of disseminated candidiasis, reflecting a parallel increase in the frequency of candidemia. This increase is multifactorial in origin and reflects increased recognition of the fungus, a growing population of patients at risk (eg, patients undergoing complex surgical procedures, patients with indwelling vascular devices), and the improved survival rates among patients with underlying neoplasms or collagen-vascular disease and patients who are immunosuppressed.
International
Similar rates of mucocutaneous and systemic candidiasis/candidemia have been observed worldwide.[10, 11] In fact, throughout the world, Candida species have replaced Cryptococcus species as the most common fungal pathogens affecting immunocompromised hosts
Mortality/Morbidity
- Mucocutaneous candidiasis: Most candidal infections are mucocutaneous and, as such, do not cause mortality. However, in patients with advanced immunodeficiency due to HIV infection, these mucosal infections can become refractory to antifungal therapy and may lead to severe oropharyngeal and esophageal candidiasis that initiates a vicious cycle of poor oral intake, malnutrition, wasting, and early death.
- Candidemia and disseminated candidiasis: Mortality rates associated with these infections have not improved markedly over the past few years and remain in the range of 30-40%. Systemic candidiasis causes more case fatalities than any other systemic mycosis. More than a decade ago, investigators reported the enormous economic impact of systemic candidiasis in hospitalized patients. Candidemia is associated with considerable prolongation in hospital stays (70 d vs 40 d in comparable patients without fungemia). Although mucocutaneous fungal infections, such as oral thrush and Candidaesophagitis, are extremely common in patients with AIDS, candidemia and disseminated candidiasis are uncommon.
Sex
Neither sex is predisposed to candidal colonization; however, VVC is the second most common cause of vaginitis in women.
Age
Persons at the extremes of age (neonates and adults >65 y) are most susceptible to candidal colonization. Mucocutaneous candidiasis is also more prevalent in neonates and older adults. Very-low-birth-weight and extremely-low-birth-weight infants are at high risk for blood culture–proven late-onset candidiasis (defined as sepsis that develops after age 72 h).[12]
History
Candidiasis can cause a wide spectrum of clinical syndromes, as described below. The clinical presentation can vary depending on the type of infection and the degree of immunosuppression.
Cutaneous candidiasis syndromes
- Generalized cutaneous candidiasis: This is an unusual form of cutaneous candidiasis that manifests as a diffuse eruption over the trunk, thorax, and extremities. The patient has a history of generalized pruritus, with increased severity in the genitocrural folds, anal region, axillae, hands, and feet. Physical examination reveals a widespread rash that begins as individual vesicles that spread into large confluent areas.
- Intertrigo: The patient has a history of intertrigo affecting any site in which skin surfaces are in close proximity, providing a warm and moist environment. A pruritic red rash develops. Physical examination reveals a rash that begins with vesiculopustules that enlarge and rupture, causing maceration and fissuring. The area involved has a scalloped border with a white rim consisting of necrotic epidermis that surrounds the erythematous macerated base. Satellite lesions are commonly found and may coalesce and extend into larger lesions (see image below).
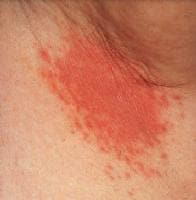 Erythema, maceration, and satellite pustules in the axilla, accompanied by soreness and pruritus, result in a form of intertrigo. Courtesy of Matthew C. Lambiase, DO.
Erythema, maceration, and satellite pustules in the axilla, accompanied by soreness and pruritus, result in a form of intertrigo. Courtesy of Matthew C. Lambiase, DO. - Metastatic skin lesions: Characteristic skin lesions occur in approximately 10% of patients with disseminated candidiasis and candidemia. The lesions may be numerous or few and are generally described as erythematous, firm, nontender macronodular lesions with discrete borders. Biopsy specimens of these lesions demonstrate yeast cells, hyphae, or pseudohyphae, and cultures are positive forCandida species in approximately 50% of cases.
- Candidafolliculitis: The infection is found predominantly in the hair follicles and, rarely, can become extensive.
- Paronychia and onychomycosis: Paronychia and onychomycosis are frequently associated with immersion of the hands in water and with diabetes mellitus. The patient has a history of a painful and erythematous area around and underneath the nail and nail bed. Physical examination reveals an area of inflammation that becomes warm, glistening, tense, and erythematous and may extend extensively under the nail. It is associated with secondary nail thickening, ridging, discoloration, and occasional nail loss.
Chronic mucocutaneous candidiasis
Chronic mucocutaneous candidiasis describes a group of Candida infections of the skin, hair, nails, and mucous membranes that tends to have a protracted and persistent course.
- History: Most infections begin in infancy or during the first 2 decades of life; onset in people older than 30 years is rare.
- Most patients survive for prolonged periods and rarely experience disseminated fungal infections. The most common cause of death is bacterial sepsis.
- Chronic mucocutaneous candidiasis is frequently associated with endocrinopathies, such as the following:
- Hypoparathyroidism
- Addison disease
- Hypothyroidism
- Diabetes mellitus
- Autoimmune antibodies to adrenal, thyroid, and gastric tissues (approximately 50%)
- Thymomas
- Dental dysplasia
- Polyglandular autoimmune disease
- Antibodies to melanin-producing cells
- Physical examination: Findings reveal disfiguring lesions of the face, scalp, hands, and nails. This is occasionally associated with oral thrush (see image below)and vitiligo.
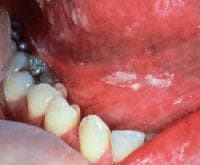 White plaques are present on the buccal mucosa and the undersurface of the tongue and represent thrush. When wiped off, the plaques leave red erosive areas. Courtesy of Matthew C. Lambiase, DO.
White plaques are present on the buccal mucosa and the undersurface of the tongue and represent thrush. When wiped off, the plaques leave red erosive areas. Courtesy of Matthew C. Lambiase, DO.
Gastrointestinal tract candidiasis
- Oropharyngeal candidiasis
- The patient usually has a history of HIV infection, wears dentures, has diabetes mellitus, or has been exposed to broad-spectrum antibiotics or inhaled steroids. Patients are frequently asymptomatic. However, some of the symptoms may include the following:
- Sore and painful mouth
- Burning mouth or tongue
- Dysphagia
- Whitish thick patches on the oral mucosa
- Physical examination reveals a diffuse erythema and white patches that appear on the surfaces of the buccal mucosa, throat, tongue, and gums. The following are the 5 types of oropharyngeal candidiasis (OPC):
- Membranous candidiasis: This is one of the most common types and is characterized by creamy-white curdlike patches on the mucosal surfaces.
- Erythematous candidiasis: This is associated with an erythematous patch on the hard and soft palates.
- Chronic atrophic candidiasis (denture stomatitis): This type is also thought to be one of the most common forms of the disease. The presenting signs and symptoms include chronic erythema and edema of the portion of the palate that comes into contact with dentures.
- Angular cheilitis: An inflammatory reaction, this type is characterized by soreness, erythema, and fissuring at the corners of the mouth (see image below).
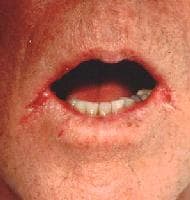 Soreness and cracks at the lateral angles of the mouth (angular cheilitis) are a frequent expression of candidosis in elderly individuals. Courtesy of Matthew C. Lambiase, DO.
Soreness and cracks at the lateral angles of the mouth (angular cheilitis) are a frequent expression of candidosis in elderly individuals. Courtesy of Matthew C. Lambiase, DO. - Mixed: A combination of any of the above types is possible.
- The patient usually has a history of HIV infection, wears dentures, has diabetes mellitus, or has been exposed to broad-spectrum antibiotics or inhaled steroids. Patients are frequently asymptomatic. However, some of the symptoms may include the following:
- Esophageal candidiasis
- The patient's history usually includes chemotherapy, the use of broad-spectrum antibiotics or inhaled steroids, the presence of HIV infection or hematologic or solid-organ malignancy. Patients may be asymptomatic or may have one or more of the following symptoms:
- Normal oral mucosa (>50% of patients)
- Dysphagia
- Odynophagia
- Retrosternal pain
- Epigastric pain
- Nausea and vomiting
- Physical examination almost always reveals oral candidiasis.
- The patient's history usually includes chemotherapy, the use of broad-spectrum antibiotics or inhaled steroids, the presence of HIV infection or hematologic or solid-organ malignancy. Patients may be asymptomatic or may have one or more of the following symptoms:
- Nonesophageal gastrointestinal candidiasis
- The patient usually has a history of neoplastic disease of the gastrointestinal tract. The esophagus is the most commonly infected site, followed by the stomach. Less commonly, patients have chronic gastric ulcerations, gastric perforations, or malignant gastric ulcers with concomitant candidal infection. The small bowel is the third most common site of infection (20%). The frequency of candidal infection in the small bowel is the same as in the large bowel. Approximately 15% of patients develop systemic candidiasis.
- Physical examination findings vary depending on the site of infection. The diagnosis, however, cannot be made solely on culture results because approximately 20-25% of the population is colonized by Candida. The following symptoms may be present:
- Epigastric pain
- Nausea and vomiting
- Abdominal pain
- Fever and chills
- Abdominal mass (in some cases)
Respiratory tract candidiasis
The respiratory tract is frequently colonized with Candida species, especially in hospitalized patients. Approximately 20-25% of ambulatory patients are colonized with Candida species.
- Laryngeal candidiasis: This is an uncommon form of invasive candidiasis that sometimes results in disseminated infection. It is primarily seen in patients with underlying hematologic or oncologic malignancies. The patient may present with a sore throat and hoarseness. The physical examination findings are generally unremarkable, and the diagnosis is frequently made with direct or indirect laryngoscopy.
- Candida tracheobronchitis: This is also an uncommon form of invasive candidiasis. Most patients with Candida tracheobronchitis are HIV-positive or are severely immunocompromised. Most patients with Candidatracheobronchitis report fever, productive cough, and shortness of breath. Physical examination reveals dyspnea and scattered rhonchi. The diagnosis is generally made with bronchoscopy.
- Candida pneumonia: This rarely develops alone and is associated with disseminated candidiasis in rare cases. The most common form of infection is multiple lung abscesses due to the hematogenous dissemination ofCandida species. The high degree of Candida colonization in the respiratory tract greatly complicates the diagnosis of Candida pneumonia. The history reveals risk factors similar to those of disseminated candidiasis, along with reports of shortness of breath, cough, and respiratory distress. Physical examination reveals fever, dyspnea, and variable breath sounds, ranging from clear to rhonchi or scattered rales.
Genitourinary tract candidiasis
- Vulvovaginal candidiasis (VVC): This is the second most common cause of vaginitis. The patient's history includes vulvar pruritus, vaginal discharge, dysuria, and dyspareunia. Approximately 10% of women experience repeated attacks of VVC without precipitating risk factors. Physical examination findings include a vagina and labia that are usually erythematous, a thick curdlike discharge, and a normal cervix upon speculum examination.[3]
- Candida balanitis: Patients report penile pruritus along with whitish patches on the penis. Candida balanitis is acquired through direct sexual contact with a partner who has VVC. Physical examination initially reveals vesicles on the penis that later develop into patches of whitish exudate. The rash occasionally spreads to the thighs, gluteal folds, buttocks, and scrotum (see image below).
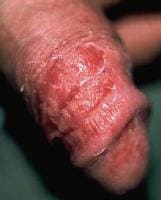 Dry, red, superficially scaly, pruritic macules and patches on the penis represent candidal balanitis. Courtesy of Matthew C. Lambiase, DO.
Dry, red, superficially scaly, pruritic macules and patches on the penis represent candidal balanitis. Courtesy of Matthew C. Lambiase, DO. - Candida cystitis: Many patients are asymptomatic. However, bladder invasion may result in frequency, urgency, dysuria, hematuria, and suprapubic pain.Candida cystitis may or may not be associated with the use of a Foley catheter. Physical examination may reveal suprapubic pain; other findings are unremarkable.
- Asymptomatic candiduria: Most catheterized patients with persistent candiduria are asymptomatic, similar to noncatheterized patients. Most patients with candiduria have easily identifiable risk factors for Candida colonization. Thus, invasive disease is difficult to differentiate from colonization based solely on culture results because approximately 5-10% of all urine cultures are positive forCandida.[13]
- Ascending pyelonephritis: The use of stents and indwelling devices, along with the presence of diabetes, is the major predisposing risk factor in ascending infection. Most patient report flank pain, abdominal cramps, nausea, vomiting, fever, chills and hematuria. Physical examination reveals abdominal pain, costovertebral-angle tenderness, and fever.
- Fungal balls: This is due to the accumulation of fungal material in the renal pelvis. The condition may produce intermittent urinary tract obstruction with subsequent anuria and ensuing renal insufficiency.
Hepatosplenic candidiasis (chronic systemic candidiasis)
Hepatosplenic candidiasis is a form of systemic candidiasis in patients with an underlying hematologic malignancy and neutropenia and develops during the recovery phase of a neutropenic episode. The patient's history includes the following:
- Fever unresponsive to broad-spectrum antimicrobials
- Right upper quadrant pain
- Abdominal pain and distension
- Jaundice (rare)
Physical examination findings include right upper quadrant tenderness and hepatosplenomegaly (< 40%).
Systemic candidiasis
Systemic candidiasis can be divided into 2 primary syndromes: candidemia and disseminated candidiasis (organ infection by Candida species). Deep organ infections due to Candida species are generally observed as part of the disseminated candidiasis syndromes and may involve one or more organs.
- Candidemia
- Candida species are currently the fourth most commonly isolated organism in blood cultures, and Candida infection is generally considered a nosocomial infection.[14, 15] The patient's history commonly reveals the following:
- Several days of fever that is unresponsive to broad-spectrum antimicrobials; frequently the only marker of infection
- Prolonged intravenous catheterization
- A history of several key risk factors (see Pathophysiology)
- Possibly associated with multiorgan infection
- Physical examination results may include the following:
- Fever
- Macronodular skin lesions (approximately 10%)
- Candidal endophthalmitis (approximately 10-28%)
- Occasionally, septic shock (hypotension, tachycardia, tachypnea)
- Other causes of candidemia without invasive disease include the following:
- Intravascular catheter-related candidiasis: This entity usually responds promptly to catheter removal and antifungal treatment.
- Suppurative thrombophlebitis: This is associated with prolonged central venous catheterization. Suppurative thrombophlebitis manifests as fever and persistent candidemia despite appropriate antifungal therapy and catheter removal. Sepsis and septic shock may develop.
- Endocarditis: The frequency of endocarditis has recently increased.[16] Candida species, primarily C albicans andCandidaparapsilosis (>60% of cases), are the most common cause of fungal endocarditis. The aortic and mitral valves are most commonly involved. The endocarditis may be exogenous (due to direct inoculation during surgery) or endogenous (due to hematogenous dissemination during bloodstream invasion.Candida endocarditis is associated with 4 main risk factors, including intravenous heroin use (frequently associated with C parapsilosis infection), chemotherapy, prosthetic valves (approximately 50%), and prolonged use of central venous catheters. The physical examination reveals a broad range of manifestations, including feverunresponsive to antimicrobials, hypotension, shock, new or changing murmurs, and large septic emboli to major organs, a characteristic of fungal endocarditis.
- Candida species are currently the fourth most commonly isolated organism in blood cultures, and Candida infection is generally considered a nosocomial infection.[14, 15] The patient's history commonly reveals the following:
- Disseminated candidiasis: This is frequently associated with multiple deep organ infections or may involve single organ infection. Unfortunately, blood cultures are negative in up to 40-60% of patients with disseminated candidiasis. The history of a patient with presumptive disseminated candidiasis reveals a fever unresponsive to broad-spectrum antimicrobials and negative results from blood culture. Physical examination reveals fever (may be the only symptom) with an unknown source and associated sepsis and septic shock.
- Candidaendophthalmitis: The two primary forms of Candida endophthalmitis are the exogenous form and the endogenous form. Exogenous endophthalmitis is associated with either accidental or iatrogenic (postoperative) injury of the eye and inoculation of the organism from the environment. Endogenous endophthalmitis results from hematogenous seeding of the eye. It has been found in 10-28% of patients with documented candidemia. Recently, newer studies have shown a decreasing incidence ofCandida endophthalmitis, possibly due to an increased awareness of this complication and the initiation of early or empirical antifungal therapy.[17] It is important to note that hematogenous candidal endophthalmitis is a marker of disseminated candidiasis.
- The patient's history reveals a broad range of manifestations, including the following.
- Eye injury
- Ophthalmic surgery
- Underlying risk factors for candidemia
- Asymptomatic and detected upon physical examination
- Ocular pain
- Photophobia
- Scotomas
- Floaters
- Physical examination reveals fever.
- Funduscopic examination reveals early pinhead-sized off-white lesions in the posterior vitreous with distinct margins and minimal vitreous haze. Classic lesions are large and off-white, similar to a cotton-ball, with indistinct borders covered by an underlying haze. Lesions are 3-dimensional and extend into the vitreous off the chorioretinal surface. They may be single or multiple.
- The patient's history reveals a broad range of manifestations, including the following.
- Renal candidiasis
- This is frequently a consequence of candidemia or disseminated candidiasis. The patient’s history includes fever that is unresponsive to broad-spectrum antimicrobials. Frequently, patients are asymptomatic and lack symptoms referable to the kidney.
- Physical examination findings are generally unremarkable, and the diagnosis is made with a urinalysis and with a renal biopsy. Otherwise, this condition is commonly diagnosed at autopsy.
- CNS infections due to Candida species
- CNS infections due to Candida species are rare and difficult to diagnose. The two primary forms of infection include the exogenous infection and the endogenous infection. The exogenous infection results from postoperative infection, trauma, lumbar puncture, or shunt placement. The endogenous infection results from hematogenous dissemination and thus involves the brain parenchyma and is associated with multiple small abscesses (eg, disseminated candidiasis).
- As with other organ infections due to Candida species, patients usually have underlying risk factors for disseminated candidiasis. CNS infections due to Candida species are frequently found in patients hospitalized for long periods in ICUs. The spectrum of this disease includes the following:
- Meningitis
- Granulomatous vasculitis
- Diffuse cerebritis with microabscesses
- Mycotic aneurysms
- Fever unresponsive to broad-spectrum antimicrobials
- Mental status changes
- Physical examination reveals the following:
- Fever
- Nuchal rigidity
- Confusion
- Coma
- Candida arthritis, osteomyelitis, costochondritis, and myositis
- Candidal musculoskeletal infections were once uncommon; recently, they have become much more common, possibly due to the increased frequency of candidemia and disseminated candidiasis. The most common sites of involvement continue to be the knee and the vertebral column. The pattern of involvement is similar to the pattern observed in bacterial infections. The infection may be divided into exogenous or endogenous forms. The exogenous infection is due to the direct inoculation of the organisms, such as postoperative infection or trauma. Affected sites include the following:
- Ribs and leg bones (patients < 20 y)
- Vertebral column and paraspinal abscess (adulthood)
- Flat bones (any age group)
- Sternum - Generally observed postoperatively after cardiac surgery
- The patient is frequently asymptomatic, and the history reveals risk factors typical of disseminated candidiasis, as well as pain localized over the affected site. The physical examination findings are frequently unremarkable but may reveal tenderness over the involved area, erythema, and bone deformity, occasionally in association with a draining fistulous tract.
- Arthritis: Candida arthritis is generally a complication of disseminated candidiasis but may be caused by trauma or direct inoculation due to surgery or steroid injections. Most cases are acute and begin as a suppurative synovitis. A high percentage of cases progress to osteomyelitis. In addition, Candida arthritis after joint replacement is not uncommon.
- Osteomyelitis: Candida osteomyelitis originates either exogenously or endogenously. The exogenous infection is due to direct inoculation of the organisms via routes such as postoperative infection, trauma, or steroid injections. The endogenous form is a complication of candidemia or disseminated candidiasis. In most cases due to hematogenous seeding, the vertebral disks are involved and frequently progress to discitis with contiguous extension into the vertebrae body. Other bones affected include the wrist, femur, scapula, and proximal humerus.
- Costochondritis: This is an uncommon form of infection and also has two modes of infection. Candida costochondritis is usually due to hematogenous infection spread or direct inoculation during surgery (median sternotomy). Costochondritis is frequently associated with pain localized over the involved area.
- Myositis: Candida myositis is uncommon but is frequently associated with disseminated candidiasis. Most patients are neutropenic and report muscular pain.
- Candidal musculoskeletal infections were once uncommon; recently, they have become much more common, possibly due to the increased frequency of candidemia and disseminated candidiasis. The most common sites of involvement continue to be the knee and the vertebral column. The pattern of involvement is similar to the pattern observed in bacterial infections. The infection may be divided into exogenous or endogenous forms. The exogenous infection is due to the direct inoculation of the organisms, such as postoperative infection or trauma. Affected sites include the following:
- Myocarditis-pericarditis: This infection is usually due to direct hematogenous spread in association with candidemia and is rarely due to the direct extension from the sternum or the esophagus. Myocarditis-pericarditis occurs as diffuse abscesses scattered throughout the myocardium surrounded by normal cardiac tissue. In patients with disseminated candidiasis, the rate of Candida myocarditis-pericarditis has been documented as high as 50%. The patient history reveals serious complications in 10-20% of cases without valvular disease. Physical examination reveals fever, hypotension, shock, tachycardia, and new murmurs or rubs (or recent changes in previously detected murmurs).
- Candida peritonitis[18]
- The patient history frequently reveals an association with gastrointestinal tract surgery, viscous perforation, or peritoneal dialysis. Candida peritonitis tends to remain localized, disseminating into the bloodstream in only 15% of cases. The range of manifestations is broad and includes fever and chills, abdominal pain and cramping, nausea, vomiting, and constipation. The isolation of Candida species from the peritoneal fluid in surgical patients needs to be carefully evaluated.
- Physical examination may reveal the following:
- Fever
- Abdominal distention
- Abdominal pain
- Absent bowel sounds
- Rebound tenderness
- Localized mass
- Candidasplenic abscess and hypersplenism: Both are manifestations of disseminated candidiasis and are usually simultaneously associated with liver involvement. Manifestations of hypersplenism are common (see Hepatosplenic candidiasis).
- Candida cholecystitis: This is uncommon and is generally associated with bacterial cholangitis and ascending cholangitis. In general, Candidacholecystitis is diagnosed at the time of surgery when a culture is obtained.
Physical
See History for physical examination findings paired with clinical syndromes.Causes
Over 200 species of Candida exist in nature; thus far, only a few species have been associated with disease in humans.- The medically significant Candida species include the following:[19]
- C albicans, the most common species identified (50-60%)
- Candida glabrata (previously known as Torulopsis glabrata) (15-20%)
- C parapsilosis (10-20%)
- Candida tropicalis (6-12%)
- Candida krusei (1-3%)
- Candida kefyr (< 5%)
- Candida guilliermondi (< 5%)
- Candida lusitaniae (< 5%)
- Candida dubliniensis, primarily recovered from patients infected with HIV
- C glabrata and C albicans account for approximately 70-80% of Candidaspecies recovered from patients with candidemia or invasive candidiasis. C glabrata has recently become very important because of its increasing incidence worldwide, its association with fluconazole resistance in up to 20% of clinical specimens, and its overall decreased susceptibility to other azoles and polyenes.
- C krusei is important because of its intrinsic resistance to ketoconazole and fluconazole (Diflucan); it is also less susceptible to all other antifungals, including itraconazole (Sporanox) and amphotericin B.
- Another important Candida species is C lusitaniae; although not as common as other Candida species, C lusitaniae is of clinical significance because it may be intrinsically resistant to amphotericin B, although it remains susceptible to azoles and echinocandins.
- C parapsilosis is also an important species to consider in hospitalized patients. It is especially common in infections associated with vascular catheters prosthetic devices. Additionally, in vitro analyses have shown that echinocandins have a higher minimum inhibitory concentration (MIC) againstC parapsilosis than other Candida species. The clinical relevance of this in vitro finding has yet to be determined.[20]
- C tropicalis has frequently been considered an important cause of candidemia in patients with cancer (leukemia) and in those who have undergone bone marrow transplantation.
Differential Diagnoses
- Abdominal Abscess
- Aspergillosis
- Cryptococcosis
- Sepsis, Bacterial
- Septic Shock
Differential Diagnoses
- Abdominal Abscess
- Aspergillosis
- Cryptococcosis
- Sepsis, Bacterial
- Septic Shock
Imaging Studies
- Imaging studies are not required or useful in the diagnosis of cutaneous candidiasis, oropharyngeal candidiasis (OPC), or vulvovaginal candidiasis (VVC).
- Chest radiography may be useful in differentiating a bacterial pneumonia as the cause of fever in patients who are hospitalized. Patients with bronchopneumonia due to hematogenous candidiasis usually have multilobar involvement.
- Esophagography/upper gastrointestinal studies may be useful for detecting abnormalities in the esophagus and stomach. Unfortunately, these studies are not helpful in determining the microbiologic etiology of the infection.
- Ultrasonography may be useful for diagnosing hepatosplenic abscess. The classic bull's eye or target lesions are observed in the liver and spleen.
- Echogenic foci with degrees of shadowing
- Intra-abdominal abscess formation
- Cholelithiasis
- Renal abscess
- Renal fungus balls
- CT scanning with contrast enhancement may be useful for diagnosing the following:
- Hepatosplenic candidiasis
- Intra-abdominal abscess or peritonitis
- Renal abscess
- Pyelonephritis
- Echocardiography may be useful for excluding or including Candida endocarditis as a possible diagnosis. It is extremely useful because fungal endocarditis is frequently associated with large vegetations that are easily observed on standard echocardiograms.
Procedures
- In patients with candidemia or disseminated candidiasis, obtaining a tissue biopsy of the involved areas is frequently helpful in establishing the presence ofCandida infection and invasion.
- Bronchoscopy with bronchoalveolar lavage and transbronchial biopsy provide adequate tissue for diagnosis of pulmonary candidiasis.
- Endoscopy provides direct examination of the esophagus and stomach, one of the organ systems most commonly infected with Candida species. It is also necessary for excluding other causes of esophagitis.
- Echocardiography may be useful for excluding or including Candida species as a cause of endocarditis. It is extremely useful because fungal endocarditis is frequently associated with large vegetations that are easily observed using standard echocardiography.
Histologic Findings
Fixed tissues can be stained with hematoxylin and eosin. In addition, fungal hyphae may be demonstrated with Grocott silver-methenamine, methylene blue, or periodic acid-Schiff staining. The classic appearance demonstrates the Candidaspecies as either round or ovoid yeast cells, hyphae, or pseudohyphae.Medical Care
The treatments used to manage Candida infections vary substantially and are based on the anatomic location of the infection, the patients' underlying disease and immune status, the patients' risk factors for infection, the specific species ofCandida responsible for infection, and, in some cases, the susceptibility of theCandida species to specific antifungal drugs.There have been significant changes in the management of candidiasis in the last few years, particularly related to the appropriate use of echinocandins and expanded-spectrum azoles for candidemia, other forms of invasive candidiasis, and mucosal candidiasis. Newer updated guidelines were published in March 2009 by the Infectious Disease Society of America (IDSA),[26] replacing a previous version from 2004.[27] These latest recommendations include the echinocandins caspofungin, micafungin, and anidulafungin, along with voriconazole and posaconazole, as well as lipid formulations of amphotericin B in various situations. Fluconazole is still considered a first-line agent in nonneutropenic patients with candidemia or suspected invasive candidiasis. However, a post-hoc analysis of clinical trial data comparing anidulafungin with fluconazole for treatment of invasive candidiasis found that anidulafungin was more effective in treating severely ill patients.[28] A revision of data outcomes ontreatment of invasive candidiasis in clinical trials appears to favor use of echinocandins in terms of increased rate of survival. This type of finding may have an impact on future treatment recommendations and strategies of drug use for invasive candidasis in different groups of patients.[29, 30]The therapeutic options available for the management of invasive candidiasis and candidemia have continued to increase with the addition of newer echinocandins[31, 32] and triazoles.- Cutaneous candidiasis: Most localized cutaneous candidiasis infections may be treated with any number of topical antifungal agents (eg, clotrimazole, econazole, ciclopirox, miconazole, ketoconazole, nystatin). If the infection is a paronychia, the most important aspect of therapy is drainage of the abscess, followed by oral antifungal therapy with either fluconazole or itraconazole. In cases of extensive cutaneous infections, infections in immunocompromised patients, folliculitis, or onychomycosis, systemic antifungal therapy is recommended. For Candida onychomycosis, oral itraconazole (Sporanox) appears to be most efficacious. Two treatment regimens are available: the daily dose of itraconazole taken for 3-6 months or the pulsed-dose regimen that requires a slightly higher daily dose for 7 days, followed by 3 weeks of no drug administration. The cycle is repeated every month for 3-6 months.
- Gastrointestinal candidiasis
- Oropharyngeal candidiasis
- Oropharyngeal candidiasis OPC can be treated with either topical antifungal agents (eg, nystatin, clotrimazole, amphotericin B oral suspension) or systemic oral azoles (fluconazole, itraconazole, or posaconazole).
- Infections in HIV-positive patients tend to respond more slowly and, in approximately 60% of patients, recur within 6 months of the initial episode. Approximately 3-5% of patients with advanced HIV infection (CD4 cell counts < 50/µL) may develop refractory OPC. In these situations, in addition to attempting correction of the immune dysfunction with HAART, higher doses of fluconazole (up to 800 mg/d) or itraconazole (up to 600 mg/d) can be attempted. Posaconazole suspension at 400 mg orally twice per day has also yielded excellent results in such patients. Additionally, caspofungin 50 mg/d IV and anidulafungin 100 mg/d IV have also yielded excellent efficacy in such patents. Amphotericin B is rarely necessary to treat such cases, but, when used, low doses of amphotericin B can be used (0.3-0.7 mg/kg) and have been shown to be effective.
- Candida esophagitis requires systemic therapy with fluconazole for 14-21 days. Parenteral therapy with fluconazole may be required initially if the patient is unable to take oral medications. Daily suppressive antifungal therapy with fluconazole 100-200 mg/d is effective for preventing recurrent episodes, but it should be used only if the recurrences become frequent or are associated with malnutrition due to poor oral intake and wasting syndrome. Recommended alternatives for fluconazole-refractory disease include itraconazole, voriconazole, caspofungin, micafungin, anidulafungin, and amphotericin B.
- Oropharyngeal candidiasis
- Genitourinary tract candidiasis
- Vulvovaginal candidiasis (VVC) can be managed with either topical antifungal agents or a single dose of oral fluconazole.[4] A single dose of oral fluconazole (150 mg) in acute episodes of VVC has been shown to yield clinical and microbiological efficacy as good as or better than topical antifungal agents. A small percentage (< 5%) of women experience chronic recurrent VVC infections, which often require long-term or prophylactic oral azole therapy for control. In such patients, the recommended regimen includes fluconazole 150 mg every other day for 3 doses, followed by weekly fluconazole 150-200 mg for 6 months.[3]This regimen prevents further recurrence in more than 80% of women.
- For asymptomatic candiduria, therapy generally depends on the presence or absence of an indwelling Foley catheter. Candiduria frequently resolves by simply changing the Foley catheter (20-25% of patients). Thus, most experts agree that asymptomatic candiduria associated with a Foley catheter does not require treatment in most cases. However, eradicating candiduria prior to any form of instrumentation or urological manipulation is prudent.
- Candida cystitis in noncatheterized patients should be treated with fluconazole at 200 mg/d orally for at least 10-14 days.
- For Candida cystitis in catheterized patients, the first step is always to remove the nidus of infection. Thus, the Foley catheter should be removed or replaced prior to initiating antifungal therapy. If the candiduria persists after the catheter change, then patients can be treated with 200 mg/d of fluconazole orally for 14 days. Alternative therapy includes amphotericin B bladder irrigation. However, its use for the treatment of funguria is significantly limited, primarily because of the required maintenance of a urinary catheter; lack of adequate studies to define the dose, duration, and method of administration; restriction of its use to uncomplicated lower urinary tract infections; and the availability of more convenient treatment options (eg, oral fluconazole therapy). The use of amphotericin B bladder irrigation is rarely needed. Administering intravenous amphotericin B to treat candiduria is rarely necessary.
- Renal candidiasis: Regardless of whether the infection involves hematogenous dissemination to the kidney or ascending infection (pyelonephritis), systemic antifungal therapy is required. The most recent comparative studies indicate that fluconazole at 400 mg/d intravenously or orally for a minimum of 2 weeks is as effective as amphotericin B without the toxicities normally associated with amphotericin B. For amphotericin B, the daily dose is 0.5-0.7 mg/kg intravenously for a total dose of 1-2 g administered over a 4- to 6-week period.
- Candidemia: This requires treatment in all patient populations. Current recommendations depend on the presence or absence of neutropenia.[26]
- In patients without neutropenia, fluconazole is the drug of choice in most cases of candidemia and disseminated candidiasis. Studies conducted by the MSG have demonstrated that fluconazole at a dose of 400 mg/d is as efficacious as amphotericin B. In addition, fluconazole has several advantages, including lower nephrotoxicity rates (< 2%) and ease of use because of the high degree of bioavailability and the long half-life of the drug.[33] Thus, once the gastrointestinal tract is functional, the parenteral antifungal may be switched to the oral formulation with the same efficacy. Alternative options listed below need to be considered depending on history of previous exposure to antifungals, the probability of fluconazole resistance according to the species of Candida recovered, the presence of comorbid conditions, and the clinical status of the patient.[34]
- An echinocandin is recommended for candidemia in most patients with neutropenia. Fluconazole is an alternative in patients who are less critically ill and who have no recent azole exposure. Voriconazole can be used when additional mold coverage is desired.
- The standard recommended dose for fluconazole is 800 mg as the loading dose, followed by fluconazole at a dose of 400 mg/d for at least 2 weeks of therapy after a demonstrated negative blood culture result or clinical signs of improvement. This treatment regimen can be used for infections due to C albicans, C tropicalis, C parapsilosis, C kefyr, C dubliniensis, C lusitaniae, and C guilliermondi.
- A critical component in the management of candidemia and disseminated candidiasis is the removal of the focus of infection, such as intravenous and Foley catheters.
- Available echinocandins for candidemia include the following:
- Caspofungin (Cancidas) can be initiated as a 70-mg loading dose, followed by 50 mg/d intravenously to complete a minimum of 2 weeks of antifungals after improvement and after blood cultures have cleared. Caspofungin is a broad-spectrum semisynthetic echinocandin. It is an effective alternative for severe mucosal infections and systemic infections due toCandida, especially those due to non-albicans Candida species such as C glabrata.
- Anidulafungin can be initiated as a 200-mg loading dose, followed by 100 mg intravenously to complete a minimum of 2 weeks of antifungals after improvement and after blood cultures have cleared. Anidulafungin is a broad-spectrum echinocandin. It is an effective alternative for severe mucosal infections and systemic infections due to Candida, especially those due to non-albicans Candida species such as C glabrata.[35]
- Micafungin can be administered at 100 mg/d intravenously to complete a minimum of 2 weeks of antifungals after improvement and after blood cultures have cleared. Micafungin is a broad-spectrum echinocandin. It has been shown to be an effective alternative for severe mucosal infections and systemic infections due to Candida, especially those due to non-albicans Candidaspecies such as C glabrata.[36]
- Additional options for candidemia include the following:
- Voriconazole can be initiated at 6 mg/kg intravenously or orally twice per day, followed by 3 mg/kg orally twice per day or 200 mg orally twice per day. Based on the findings from a global multicenter clinical trial, voriconazole has also been approved for use in candidemia in patients who are not neutropenic.[37]
- Amphotericin B deoxycholate can be administered at 0.7 mg/kg/d intravenously for a total dose of 1-2 g over a 4- to 6-week period.
- Liposomal preparations of amphotericin B have comparable efficacy to conventional amphotericin B, but renal toxicity is considerably less common with the former.
- Chronic mucocutaneous candidiasis: This condition is generally treated with oral azoles, such as fluconazole at a dose of 100-400 mg/d or itraconazole at a dose of 200-600 mg/d until the patient improves. The initial therapy for acute infection is always followed by maintenance therapy with the same azole for life.
- Hepatosplenic candidiasis: Induction therapy is initially started with amphotericin B deoxycholate for at least 2 weeks, followed by consolidation therapy with fluconazole at a dose of 400 mg/d for an additional 4-12 weeks depending on the response.
- Respiratory tract candidiasis: If the diagnosis is established based on biopsy findings, then the infection is treated as disseminated candidiasis.
- Empirical treatment options for suspected invasive candidiasis include the following:
- Empirical antifungal therapy should be considered for critically ill patients with risk factors for invasive candidiasis and no other cause of fever, and it should be based on clinical assessment of risk factors, serologic markers for invasive candidiasis, and/or culture data from nonsterile sites. (Its benefits have not been clearly determined.)[38]
- This continues to be a problematic decision since criteria for starting empirical antifungal therapy remain poorly defined. Empirical therapy in persistently febrile and neutropenic patients should cover infections caused by yeasts and molds.
- The choice of drugs in nonneutropenic patients is similar to that for proven candidiasis. Recommended agents include fluconazole or an echinocandin.
- In neutropenic patients, a lipid formulation of amphotericin B, caspofungin, or voriconazole is recommended. Azoles should not be used for empirical therapy in individuals who have received an azole for prophylaxis.
- Disseminated candidiasis with end organ infection: Disseminated candidiasis with end organ involvement requires an individualized approach. Thus, the manifestation of invasive candidiasis involving localized structures, such as inCandida osteomyelitis, arthritis, endocarditis, pericarditis, and meningitis, requires prolonged antifungal therapy for at least 4-6 weeks. The optimum dosage and duration of therapy for various types of deep candidal infection have not been definitively determined.
- The standard recommended dose for most Candida infections is fluconazole at 800 mg as the loading dose, followed by fluconazole at a dose of 400 mg/d either intravenously or orally for at least 2 weeks of therapy after a demonstrated negative blood culture result or clinical signs of improvement.
- The echinocandins have become first-line therapy for this type of infection in many situations because of their efficacy and low incidence of adverse events and drug interactions.
- Caspofungin (Cancidas)[39] can be initiated as a 70-mg loading dose, followed by 50 mg/d intravenously to complete a minimum of 2 weeks of antifungals after improvement and after blood cultures have cleared. Caspofungin is a broad-spectrum semisynthetic echinocandin. It is an effective alternative for severe mucosal infections and systemic infections due toCandida, especially those due to non-albicans Candida species such as C glabrata.
- Anidulafungin can be initiated as a 200-mg loading dose, followed by 100 mg intravenously to complete a minimum of 2 weeks of antifungals after improvement and after blood cultures have cleared. Anidulafungin is a broad-spectrum echinocandin. It is an effective alternative for severe mucosal infections and systemic infections due to Candida, especially those due to non-albicans Candida species such as C glabrata.[35]
- Micafungin can be administered at 100 mg/d intravenously to complete a minimum of 2 weeks of antifungals after improvement and after blood cultures have cleared. Micafungin is a broad-spectrum echinocandin. It has been shown to be an effective alternative for severe mucosal infections and systemic infections due to Candida, especially those due to non-albicans Candidaspecies such as C glabrata.[36]
- Voriconazole can be initiated at 6 mg/kg intravenously or orally twice per day, followed by 3 mg/kg orally twice per day or 200 mg orally twice per day. Based on the findings from a global multicenter clinical trial, voriconazole has also been approved for use in candidemia in patients who are not neutropenic.[37]
- Amphotericin B deoxycholate has been an alternative to fluconazole for many years. However, with the advent of the newer azoles and the echinocandins, its role as a primary or secondary option needs to be reconsidered. The dose for amphotericin B deoxycholate is 0.5-0.7 mg/kg/d intravenously to achieve a minimum of 1- to 2-g total dose. For the treatment of invasive candidiasis caused by less-susceptible species, such as C glabrata and C krusei, higher doses (up to 1 mg/kg/d) should be considered.
- Liposomal preparations of amphotericin B are recommended at doses between 3 and 5 mg/kg/d when used for invasive candidiasis.
- Special situations involving antifungal resistance: Several of the Candidaspecies require special mention because of their known intrinsic resistance to antifungals.
- Because C glabrata is known to be resistant to fluconazole in 15-25% of cases and has decreased susceptibility to most antifungals, C glabrata infections require a change in conventional antifungal therapy. The drugs of choice for such infections are the echinocandins: caspofungin 70 mg intravenously as a loading dose, followed by 50 mg/d; anidulafungin 200-mg loading dose, followed by 100 mg/d; or micafungin 100 mg/day intravenously. An alternative is voriconazole at 6 mg/kg administered twice on the first day, followed by 3 mg/kg twice per day or 200 mg twice per day orally; other options include amphotericin B deoxycholate (1 mg/kg/d), or lipid preparations of amphotericin B at 3-5 mg/kg/d.
- If in vitro susceptibility assays are available, it may be worthwhile to establish the in vitro susceptibility of the C glabrata strain to fluconazole. If the MIC is less than 8 μg/mL, then fluconazole can be used at 400 mg/d intravenously or orally.
- C krusei infections necessitate the use of an agent other than fluconazole because this organism is intrinsically resistant to fluconazole and has a decreased susceptibility to itraconazole, ketoconazole, and amphotericin B. Thus, the preferred regimen includes echinocandins (caspofungin, anidulafungin, or micafungin) voriconazole, or amphotericin B at 1 mg/kg/d. Infections due to C lusitaniae or C guilliermondi necessitate the use of fluconazole, voriconazole, or the echinocandins because these isolates are frequently intrinsically resistant to amphotericin B or develop resistance to amphotericin B while the patient is on therapy.
- Alternative antifungal regimens
- Alternative regimens may be considered in patients who are intolerant to the treatment regimens or when the infection is refractory to the antifungal regimen. The combination of amphotericin B and flucytosine has been recommended in several special situations. For instance, this combination has been used in immunocompromised patients with endophthalmitis, meningitis, or osteomyelitis. Flucytosine appears to interact synergistically with amphotericin B in animal models.
- The role of other combinations of antifungals to treat complicatedCandida infections needs to be evaluated. A human recombinant monoclonal antibody against heat shock protein 90 was recently reported to significantly improve outcomes in patients treated with lipid-associated amphotericin B for confirmed invasive candidiasis.[40]However,
Surgical Care
.- Major organ infections associated with candidal abscess formation may require surgical drainage procedures along with the appropriate antifungal therapy.
- Prosthetic joint infection with Candida species requires the removal of the prosthesis.
- Surgical debridement is generally necessary for sternal infections and frequently for vertebral osteomyelitis.
- Splenic abscesses occasionally require splenectomy.
- Valve replacement surgery is always indicated to treat endocarditis.
- In addition to medical management, vitrectomy is a therapeutic option in fungal endophthalmitis.[41]
Consultations
In some forms of candidiasis, involving physicians of different specialties for some of the specific infections may be necessary. Some examples of these situations include endocarditis, endophthalmitis, peritonitis, osteomyelitis, and other forms of invasive candidiasis that may require surgical drainage and debridement.- Ophthalmologist
- General surgeon
- Cardiothoracic surgeon
- Gastroenterologist
- Infectious disease specialist
- Orthopedic surgeon
Medication Summary
Successful therapy for serious systemic Candida infections requires initiation of antifungal therapy as early as possible, as soon as adequate culture results are obtained.Different classes of antifungals are now available to manage any type of candidal infection. Azoles, fluconazole in particular,[33] have become the mainstay of therapy over the past few years. These include topical and systemic agents. Posaconazole is the most recent addition to this group of antifungals. Polyenes include amphotericin B, liposomal amphotericin B formulations, and topical nystatin. Allylamines include terbinafine, which is formulated in a topical preparation and an oral tablet. The newest group of antifungals is echinocandins, including caspofungin, micafungin, and anidulafungin. These drugs have shown excellent clinical efficacy, a low incidence of adverse events, a good safety profile, and ease of use.Azole Antifungals
Class Summary
These agents are synthetic compounds that include 2 groups, imidazoles and triazoles. Triazoles have 3 atoms of nitrogen in the azole ring. Imidazoles have only two. The primary mechanism of action is inhibition of lanosterol 14-alpha-demethylase, an enzyme required for the synthesis of ergosterol, the main component of fungal cell membranes. Imidazole agents include miconazole, ketoconazole, and clotrimazole. Triazole agents, which are now the most commonly used azoles, include fluconazole, itraconazole, econazole, terconazole, butoconazole, and tioconazole. Newer triazoles (ie, voriconazole, posaconazole, ravuconazole) are active against fluconazole-resistant strains ofCandida. Voriconazole and posaconazole have shown high efficacy against candidiasis in recent clinical trials.[44, 37, 45]Topical agents are frequently used as front-line agents to manage localized or superficial forms of candidiasis such as cutaneous candidiasis, oropharyngeal candidiasis (OPC), and vulvovaginal candidiasis (VVC). These preparations are available as a cream for topical use, as troches for OPC, and as a vaginal suppositories or tablets for vaginitis.Fluconazole (Diflucan)
Triazole with less effect on human sterol metabolism. Does not decrease cortisol and testosterone levels, as occurs with ketoconazole. Has fewer adverse effects and better tissue distribution than older systemic imidazoles. Available PO/IV and has demonstrated efficacy in topical and invasive forms of candidiasis. Available in 50-, 100-, 150-, and 200-mg tabs.Daily dose varies with indication.Itraconazole (Sporanox)
Has fungistatic activity. Synthetic triazole antifungal agent that slows fungal cell growth by inhibiting cytochrome P450-dependent synthesis of ergosterol, a vital component of fungal cell membranes. Effective against broad range of fungi, including Candida species, and is indicated for treatment of cutaneous, oral, esophageal, and disseminated candidiasis.Available IV, 100-mg caps, and oral solution at 10 mg/mL.Caps require gastric acidity for absorption and should be taken with food to increase absorption. Liquid formulation increases bioavailability and decreases need for acidity for proper absorption.Use of solution has been recommended in mucosal and invasive candidiasis, while caps can be used in onychomycosis and dermatophyte infections.Voriconazole (Vfend)
Available as tablet, suspension and parenteral preparations. Effective as fluconazole against esophageal candidiasis, and as effective as amphotericin B deoxycholate in treatment of candidemia and invasive candidiasis. In Europe, it has been approved for "treatment of fluconazole-resistant serious invasive Candidainfections (including C krusei)." Additionally, associated with fewer breakthrough fungal infections when used as empiric therapy in febrile neutropenic patients. FDA approved for esophageal candidiasis and candidemia.Posaconazole (Noxafil)
Novel triazole antifungal agent. Blocks ergosterol synthesis of cell membrane by inhibiting enzyme lanosterol 14-alpha-demethylase and sterol precursor accumulation. Action results in cell membrane disruption. Available as oral susp (200 mg/5 mL). Approved for treatment of OPC, including OPC refractory to itraconazole and/or fluconazole and for prophylaxis of infections due to Candidaand Aspergillus in patients who are at high risk, such as those undergoing stem cell transplants with graft versus host disease or with prolonged neutropenia due to a hematologic malignancy or its treatment.Glucan synthesis inhibitors (echinocandins)
Class Summary
These agents inhibit the formation of fungal cell wall. The antifungal class has expanded with the approvals of caspofungin, micafungin, and anidulafungin. Indications are evolving but have been approved for complicated forms of invasive candidiasis, candidemia, disease refractory to other systemic antifungals, and intolerance to amphotericin B. They are broad spectrum and fungicidal against most Candida species, except C parapsilosis and C guilliermondii.Caspofungin (Cancidas)
FDA approved to treat candidemia, invasive candidiasis, and esophageal candidiasis. Initially approved to treat refractory invasive aspergillosis. Also approved as empiric therapy for presumed fungal infections in febrile neutropenic patients. First of a new class of antifungal drugs (glucan synthesis inhibitors). Inhibits synthesis of (1,3) β -D-glucan, an essential component of fungal cell wall. This component is not found in mammalian cell walls.Micafungin (Mycamine)
A member of a new class of antifungal agents, echinocandins, that inhibit cell wall synthesis. Echinocandins inhibit the synthesis of (1,3)-β -D-glucan, an essential component of the fungal cell wall, not present in mammalian cells. Indications include prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation, treatment of esophageal candidiasis, candidemia, and invasive candidiasis.Anidulafungin (Eraxis)
One of the newer antifungal agents belonging to the echinocandin class. Also inhibits synthesis of (1,3)-β -D-glucan, an essential component of fungal cell walls. Indicated for treatment of esophageal candidiasis, candidemia, and other forms of candidal infections (eg, intra-abdominal abscesses, peritonitis).Medication Summary
Successful therapy for serious systemic Candida infections requires initiation of antifungal therapy as early as possible, as soon as adequate culture results are obtained.Different classes of antifungals are now available to manage any type of candidal infection. Azoles, fluconazole in particular,[33] have become the mainstay of therapy over the past few years. These include topical and systemic agents. Posaconazole is the most recent addition to this group of antifungals. Polyenes include amphotericin B, liposomal amphotericin B formulations, and topical nystatin. Allylamines include terbinafine, which is formulated in a topical preparation and an oral tablet. The newest group of antifungals is echinocandins, including caspofungin, micafungin, and anidulafungin. These drugs have shown excellent clinical efficacy, a low incidence of adverse events, a good safety profile, and ease of use.[42, 43]Azole Antifungals
Class Summary
These agents are synthetic compounds that include 2 groups, imidazoles and triazoles. Triazoles have 3 atoms of nitrogen in the azole ring. Imidazoles have only two. The primary mechanism of action is inhibition of lanosterol 14-alpha-demethylase, an enzyme required for the synthesis of ergosterol, the main component of fungal cell membranes. Imidazole agents include miconazole, ketoconazole, and clotrimazole. Triazole agents, which are now the most commonly used azoles, include fluconazole, itraconazole, econazole, terconazole, butoconazole, and tioconazole. Newer triazoles (ie, voriconazole, posaconazole, ravuconazole) are active against fluconazole-resistant strains ofCandida. Voriconazole and posaconazole have shown high efficacy against candidiasis in recent clinical trials.[44, 37, 45]Topical agents are frequently used as front-line agents to manage localized or superficial forms of candidiasis such as cutaneous candidiasis, oropharyngeal candidiasis (OPC), and vulvovaginal candidiasis (VVC). These preparations are available as a cream for topical use, as troches for OPC, and as a vaginal suppositories or tablets for vaginitis.Fluconazole (Diflucan)
Triazole with less effect on human sterol metabolism. Does not decrease cortisol and testosterone levels, as occurs with ketoconazole. Has fewer adverse effects and better tissue distribution than older systemic imidazoles. Available PO/IV and has demonstrated efficacy in topical and invasive forms of candidiasis. Available in 50-, 100-, 150-, and 200-mg tabs.Daily dose varies with indication.Itraconazole (Sporanox)
Has fungistatic activity. Synthetic triazole antifungal agent that slows fungal cell growth by inhibiting cytochrome P450-dependent synthesis of ergosterol, a vital component of fungal cell membranes. Effective against broad range of fungi, including Candida species, and is indicated for treatment of cutaneous, oral, esophageal, and disseminated candidiasis.Available IV, 100-mg caps, and oral solution at 10 mg/mL.Caps require gastric acidity for absorption and should be taken with food to increase absorption. Liquid formulation increases bioavailability and decreases need for acidity for proper absorption.Use of solution has been recommended in mucosal and invasive candidiasis, while caps can be used in onychomycosis and dermatophyte infections.Voriconazole (Vfend)
Available as tablet, suspension and parenteral preparations. Effective as fluconazole against esophageal candidiasis, and as effective as amphotericin B deoxycholate in treatment of candidemia and invasive candidiasis. In Europe, it has been approved for "treatment of fluconazole-resistant serious invasive Candidainfections (including C krusei)." Additionally, associated with fewer breakthrough fungal infections when used as empiric therapy in febrile neutropenic patients. FDA approved for esophageal candidiasis and candidemia.Posaconazole (Noxafil)
Novel triazole antifungal agent. Blocks ergosterol synthesis of cell membrane by inhibiting enzyme lanosterol 14-alpha-demethylase and sterol precursor accumulation. Action results in cell membrane disruption. Available as oral susp (200 mg/5 mL). Approved for treatment of OPC, including OPC refractory to itraconazole and/or fluconazole and for prophylaxis of infections due to Candidaand Aspergillus in patients who are at high risk, such as those undergoing stem cell transplants with graft versus host disease or with prolonged neutropenia due to a hematologic malignancy or its treatment.Glucan synthesis inhibitors (echinocandins)
Class Summary
These agents inhibit the formation of fungal cell wall. The antifungal class has expanded with the approvals of caspofungin, micafungin, and anidulafungin. Indications are evolving but have been approved for complicated forms of invasive candidiasis, candidemia, disease refractory to other systemic antifungals, and intolerance to amphotericin B. They are broad spectrum and fungicidal against most Candida species, except C parapsilosis and C guilliermondii.Caspofungin (Cancidas)
FDA approved to treat candidemia, invasive candidiasis, and esophageal candidiasis. Initially approved to treat refractory invasive aspergillosis. Also approved as empiric therapy for presumed fungal infections in febrile neutropenic patients. First of a new class of antifungal drugs (glucan synthesis inhibitors). Inhibits synthesis of (1,3) β -D-glucan, an essential component of fungal cell wall. This component is not found in mammalian cell walls.Micafungin (Mycamine)
A member of a new class of antifungal agents, echinocandins, that inhibit cell wall synthesis. Echinocandins inhibit the synthesis of (1,3)-β -D-glucan, an essential component of the fungal cell wall, not present in mammalian cells. Indications include prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation, treatment of esophageal candidiasis, candidemia, and invasive candidiasis.Anidulafungin (Eraxis)
One of the newer antifungal agents belonging to the echinocandin class. Also inhibits synthesis of (1,3)-β -D-glucan, an essential component of fungal cell walls. Indicated for treatment of esophageal candidiasis, candidemia, and other forms of candidal infections (eg, intra-abdominal abscesses, peritonitis).Polyenes
Class Summary
These are broad-spectrum fungicidal agents. Mechanism of action is by insertion into fungal cytoplasmic membrane, causing increases in permeability. Membrane channel activity is increased at lower doses, and pores are formed at higher concentrations.Amphotericin B (Fungizone, Amphocin)
One of the oldest antifungals, in use for more than 40 y, and the criterion standard of antifungal therapy.In recent years, lipid formulations have been developed. Total dose must be adjusted depending on type of candidal infection being treated. Most patients receive total dose of 0.5-1.5 g.Amphotericin B, lipid formulations (Amphotec, Abelcet, AmBisome)
Novel lipid formulations of amphotericin B that deliver higher concentrations of drug with a theoretical increase in therapeutic potential and decreased nephrotoxicity.Formulation types include amphotericin B lipid complex (ABLC, Abelcet), amphotericin B colloidal dispersion (ABCD, Amphotec), and liposomal amphotericin B (L-AMB, AmBisome).ABLC and ABCD approved for treating adults and children intolerant of conventional amphotericin B or with fungal infections refractory to conventional amphotericin B. L-AMB is approved for aspergillosis, candidiasis, cryptococcosis, and neutropenic patients with persistent fever on broad-spectrum antibiotics.Nystatin (Mycostatin)
Fungicidal and fungistatic antibiotic obtained from Streptomyces noursei. Effective against various yeasts and yeastlike fungi. Changes permeability of fungal cell membrane after binding to cell membrane sterols, causing cellular contents to leak. Membrane channel activity is increased at lower doses, and pores are formed at higher concentrations.Antimetabolite
Class Summary
Flucytosine is an antimetabolite originally developed for the treatment of leukemia.Flucytosine (Ancobon)
It is deaminated to 5-fluorouracil in the fungal cell by an enzyme not present in mammalian cells, and inhibits RNA and protein synthesis. Active against Candidaand Cryptococcus species and generally used in combination with amphotericin B. It has been used in studies in invasive candidiasis. Avoid use as single agent because of ability to quickly develop resistance in vivo.Topical azoles
Class Summary
These agents are used extensively to treat common mucocutaneous uncomplicated forms of candidiasis.Clotrimazole (Mycelex, Femizole-7)
Broad-spectrum antifungal agent that inhibits yeast growth by altering cell membrane permeability, causing death of fungal cells.Butoconazole (Femstat-3, Gynazole-1)
Broad-spectrum antifungal agent that inhibits yeast growth by altering cell membrane permeability, causing death of fungal cells.Use 2% vaginal cream. Available OTC.Miconazole vaginal (Monistat, Micatin)
Damages fungal cell wall membrane by inhibiting biosynthesis of ergosterol. Membrane permeability is increased, causing nutrients to leak out, resulting in fungal cell death. Lotion preferred in intertriginous areas. If cream is used, apply sparingly to avoid maceration effects.Tioconazole (Vagistat-1)
Damages fungal cell wall membrane by inhibiting biosynthesis of ergosterol. Membrane permeability is increased, causing nutrients to leak out, resulting in fungal cell death.Terconazole vaginal (Terazol-7, Terazol-3)
Damages fungal cell wall membrane by inhibiting biosynthesis of ergosterol. Membrane permeability is increased, causing nutrients to leak out, resulting in fungal cell death.Allylamines
Class Summary
These agents cause a deficiency of ergosterol within the fungal cell wall, causing fungal cell death.Terbinafine (Daskil, Lamisil)
For treatment of paronychia; allylamine antifungal, which inhibits squalene epoxidase and decreases ergosterol synthesis, causing fungal-cell death.Use medication until symptoms significantly improve.Duration of treatment should be >1 wk but not >4 wk. May not be as effective for candidal infections as azole antifungals.Further Inpatient Care
Inpatient care is frequently prolonged because of the severe nature of the disseminated infections. Antifungal therapy may be necessary for a prolonged period, either parenterally or orally.(1,3)β-D-glucan assay is a useful nonculture method for diagnosis of invasive candidasis. A decrease in levels during therapy has been associated with treatment success in patients on echinocandin therapy with proven invasive candidiasis. Consecutive serum measurements may be useful as prognostic markers of response.Further Outpatient Care
- Mucocutaneous candidiasis
- Patients treated in the outpatient area may be discharged home with medications. Instruct patients to follow up if the symptoms persist or worsen.
- If the infections are recurrent, perform an HIV antibody test and rule out conditions that produce immune suppression, such as hematologic malignancies, solid organ malignancy, and diabetes mellitus. If no etiology is established, refer the patients for consultation with an infectious disease specialist to rule out an underlying immune deficiency.
- Candidemia and disseminated candidiasis
- Because of the severity of the infections, some patients may remain hospitalized for a prolonged period.
- Patients on outpatient amphotericin B must be monitored 2-3 times weekly because of its high incidence of adverse effects. The parameters that need to be monitored include CBC count with differentials; electrolyte evaluations; and serum magnesium, BUN, and serum creatinine levels.
Inpatient & Outpatient Medications
- With newer treatment modalities that have been recently instituted, de-escalation of antifungal therapy or the rapid switch from intravenous to oral administration is encouraged. Recent clinical studies suggest that patients who are clinically stable and have a functional gastrointestinal tract on day 4-5 of parenteral intravenous antifungal administration should be switched from intravenous to oral therapy with either fluconazole or voriconazole.
- Although relatively uncommon, patients may be discharged home on parenteral antifungal therapy or oral azole therapy with close monitoring for toxicity
- Transfer patients to the service that can care for the specific candidal infections (eg, general surgery, ICU).
- Transfer patients with sepsis or altered mental status to an appropriate critical care unit.
Deterrence/Prevention
- Antifungal prophylaxis of invasive candidiasis in high-risk patients is currently recommended for the following:[26, 47]
- Stem cell transplant recipients, primarily those with allogeneic transplants, are recommended to receive fluconazole initiated 1 day prior to neutropenia and continued until neutropenia resolves. Micafungin and posaconazole are also recommended for this indication.[48]
- Solid organ transplant recipients may be considered for antifungal prophylaxis with fluconazole or liposomal amphotericin B for the prevention of candidiasis. This is recommended for postoperative antifungal prophylaxis in liver, pancreas, and small bowel transplant recipients at high risk of candidiasis. Additional indications are being investigated.[49]
- For patients with chemotherapy-induced neutropenia, fluconazole, posaconazole, or caspofungin is recommended during induction chemotherapy for the duration of neutropenia.
- Most recent candidiasis treatment guidelines recommend prophylaxis in high-risk ICU patients in adult units that have high incidence of invasive candidiasis.[26] Oral nystatin prophylaxis has been shown to decrease colonization in ICU patients and needs to be investigated as a potential strategy to control candida-related infection in appropriately selected patients in this setting.[50]
- Currently, no strong indications exist for primary or secondary prevention of oropharyngeal candidiasis (OPC) or vaginal candidiasis in patients infected with HIV. However, concern does exist about the potential development of resistance or colonization by resistant species or strains of Candida. Prophylaxis may be indicated in a select group of patients with recurrent symptomatic candidiasis.
- Control the blood glucose level in patients with diabetes mellitus.
- Eliminate or decrease risk factors such as steroids, cyclosporin, and tacrolimus.
- Nosocomial candidemia prevention should be based on hand hygiene, optimal catheter care, and prudent antimicrobial use
Complications
- If left untreated, candidemia can lead to metastatic foci of infection in the eyes, vertebral column, liver, spleen, CNS, and kidneys. Initiate prompt treatment to prevent foci of infection, abscess formation, and deat
Prognosis
- Prognosis depends on several factors, such as the site of infection, the degree and type of immunosuppression, and the rapidity of diagnosis and treatment.
- Mucocutaneous candidiasis carries an excellent prognosis, with no mortality and only minimal morbidity.
- Systemic candidiasis carries a mortality rate of 30-40% and is generally correlated with the degree of immunosuppression and the underlying disease. In certain groups of patients, the presentation of Candida infection increases the likelihood of death, lengthens hospital stays, and increases hospitalization costs.[52, 53]
- The longer the delay to initiate antifungal therapy, the higher the morbidity and mortality associated with candidemia and disseminated candidiasis.
Patient Education
- Inform patients and their families about the risk factors associated with mucosal and systemic candidiasis. In addition, inform them that the systemic form of the disease is extremely serious and is associated with high morbidity and mortality rates unless aggressive action is undertaken.
- For excellent patient education resources, visit eMedicineHealth's Infections Center, Children's Health Center, and Skin Conditions and Beauty Center. Also, see eMedicineHealth's patient education article Candidiasis (Yeast Infection),Yeast Infection Diaper Rash, and Yeast Infection Skin Rash.